Long-Term Restoration of Thymidine Phosphorylase Function and Nucleoside Homeostasis Using Hematopoietic Gene Therapy in a Murine Model of Mitochondrial Neurogastrointestinal Encephalomyopathy
- PMID: 27004974
- PMCID: PMC5079415
- DOI: 10.1089/hum.2015.160
Long-Term Restoration of Thymidine Phosphorylase Function and Nucleoside Homeostasis Using Hematopoietic Gene Therapy in a Murine Model of Mitochondrial Neurogastrointestinal Encephalomyopathy
Abstract
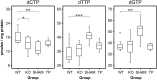
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is a metabolic disorder caused by mutations in TYMP, encoding thymidine phosphorylase (TP). In MNGIE patients, TP dysfunction produces systemic thymidine and deoxyuridine accumulation, which ultimately impairs mitochondrial DNA replication and results in mitochondrial dysfunction. To date, only allogeneic hematopoietic stem cell transplantation has demonstrated long-term clinical efficacy, but high morbidity and mortality associated with this procedure necessitate the search for safer alternatives. In a previous study, we demonstrated that hematopoietic stem cell gene therapy using a lentiviral vector containing the coding sequence of TYMP restored the biochemical homeostasis in an animal model of MNGIE. In the present follow-up study, we show that ectopic expression of TP in the hematopoietic system restores normal nucleoside levels in plasma, as well as in tissues affected in MNGIE such as small intestine, skeletal muscle, brain, and liver. Mitochondrial dNTP pool imbalances observed in liver of the animal model were also corrected by the treatment. The biochemical effects were maintained at least 20 months even with low levels of chimerism. No alterations in the blood cell counts or other toxic effects were observed in association with the lentiviral transduction or TP overexpression. These results further support the notion that gene therapy is a feasible treatment option for MNGIE.
Conflict of interest statement
Author Disclosure No competing financial interests exist.
Figures





References
-
- Hirano M, Nishigaki Y, Marti R. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): A disease of two genomes. Neurologist 2004;10:8–17 - PubMed
-
- Hirano M, Silvestri G, Blake DM, et al. . Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): Clinical, biochemical, and genetic features of an autosomal recessive mitochondrial disorder. Neurology 1994;44:721–727 - PubMed
-
- Desgranges C, Razaka G, Rabaud M, et al. . Catabolism of thymidine in human blood platelets: Purification and properties of thymidine phosphorylase. Biochim Biophys Acta 1981;654:211–218 - PubMed
-
- Marti R, Nishigaki Y, Vila MR, et al. . Alteration of nucleotide metabolism: A new mechanism for mitochondrial disorders. Clin Chem Lab Med 2003;41:845–851 - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

