Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial
- PMID: 27005405
- PMCID: PMC4973216
- DOI: 10.1038/ijo.2016.52
Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial
Abstract
Background: Obesity is strongly associated with prevalence of obstructive sleep apnea (OSA), and weight loss has been shown to reduce disease severity.
Objective: To investigate whether liraglutide 3.0 mg reduces OSA severity compared with placebo using the primary end point of change in apnea-hypopnea index (AHI) after 32 weeks. Liraglutide's weight loss efficacy was also examined.
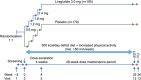
Subjects/methods: In this randomized, double-blind trial, non-diabetic participants with obesity who had moderate (AHI 15-29.9 events h(-1)) or severe (AHI ⩾30 events h(-1)) OSA and were unwilling/unable to use continuous positive airway pressure therapy were randomized for 32 weeks to liraglutide 3.0 mg (n=180) or placebo (n=179), both as adjunct to diet (500 kcal day(-1) deficit) and exercise. Baseline characteristics were similar between groups (mean age 48.5 years, males 71.9%, AHI 49.2 events h(-1), severe OSA 67.1%, body weight 117.6 kg, body mass index 39.1 kg m(-2), prediabetes 63.2%, HbA1c 5.7%).
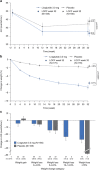
Results: After 32 weeks, the mean reduction in AHI was greater with liraglutide than with placebo (-12.2 vs -6.1 events h(-1), estimated treatment difference: -6.1 events h(-1) (95% confidence interval (CI), -11.0 to -1.2), P=0.0150). Liraglutide produced greater mean percentage weight loss compared with placebo (-5.7% vs -1.6%, estimated treatment difference: -4.2% (95% CI, -5.2 to -3.1%), P<0.0001). A statistically significant association between the degree of weight loss and improvement in OSA end points (P<0.01, all) was demonstrated post hoc. Greater reductions in glycated hemoglobin (HbA1c) and systolic blood pressure (SBP) were seen with liraglutide versus placebo (both P<0.001). The safety profile of liraglutide 3.0 mg was similar to that seen with doses ⩽1.8 mg.
Conclusions: As an adjunct to diet and exercise, liraglutide 3.0 mg was generally well tolerated and produced significantly greater reductions than placebo in AHI, body weight, SBP and HbA1c in participants with obesity and moderate/severe OSA. The results confirm that weight loss improves OSA-related parameters.
Trial registration: ClinicalTrials.gov NCT01557166.
Conflict of interest statement
Dr Blackman: consultant/advisory board participant for Novo Nordisk, Merck Canada, Valeant Canada, Paladin Labs Inc. Dr Foster: at the time of the study was a scientific advisory board participant for Con Agra Foods, United Health Group and Tate and Lyle, and a consultant to Eisai and Novo Nordisk; currently employed by Weight Watchers International. Dr Zammit: consultant for Acorda, Actelion, Alexza, Arena, Aventis, Biovail, Boehringer-Ingelheim, Cephalon, Elan, Eli Lilly, Evotec, Forest, Glaxo Smith Kline, Jazz, King Pharmaceuticals, Ligand, McNeil, Merck, Neurocrine Biosciences, Organon, Pfizer, Purdue, Renovis, Sanofi-Aventis, Select Comfort, Sepracor, Shire, Somnus, Takeda Pharmaceuticals, Vela, Wyeth; grants/research support from Abbott, Abbvie, Actelion, Ancile, Apnex, Arena, Astra-Zeneca, Aventis, Banyu, Biomarin, BMS, Catalyst, Cephalon Inc., CHDI, Elan, Epix, Eisai, Elminda, Evotec, Forest, Galderma, Genentech, Gilead, Glaxo Smith Kline, Gilead, H. Lundbeck A/S, Janssen, Johnson & Johnson, King, Merck and Co., National Institute of Health (NIH), Neurim, Neurocrine Biosciences, Naurex, Neurim, Neurogen, Novo Nordisk, Organon, Orphan Medical, Otsuka, Pfizer, Predix, Respironics, Saladax, Sanofi-Aventis, Sanofi-Synthelabo, Schering-Plough, Sepracor, Sunovion, Shire, Somaxon, Takeda Pharmaceuticals North America, Targacept, Teva, Thymon, Transcept, UCB Pharma, Ultragenyx, Predix, Vanda, Wyeth-Ayerst Research; honoraria received from Neurocrine Biosciences, King Pharmaceuticals, McNeil, Sanofi-Aventis, Sanofi-Synthelabo, Sepracor, Takeda Pharmaceuticals, Vela Pharmaceuticals, Wyeth-Ayerst Research; ownership interest in Clinilabs, Inc., Clinilabs IPA, Inc., Home Sleep and Respiratory Care, Nationwide Sleep Testing. Dr Rosenberg: received research grant support from Merck, Novo Nordisk, Pfizer, Teva, Jazz Pharmaceuticals, Sunovion Pharmaceuticals and Philips-Respironics. Dr Aronne: advisory board member for Myos Corporation; consultant, speaker, advisor, or received research support from Aspire Bariatrics Inc., Eisai, Ethicon Endo-Surgery Inc., GlaxoSmithKline Consumer Healthcare LP, GI Dynamics, Novo Nordisk, Pfizer, Takeda Pharmaceuticals, Vivus Inc., Zafgen Inc.; ownership interest in Myos Corporation and BMIQ. Dr Wadden: advisory board participant for Orexigen Pharmaceuticals, Inc., Novo Nordisk, Nutrisystem, and Shire Pharmaceuticals, with research support (to the University of Pennsylvania) from the first three companies. Dr Claudius: employee of Novo Nordisk and owns company stock. Dr Jensen: employee of Novo Nordisk and owns company stock. Dr Mignot: advisory board member, consultant for and research support from Jazz Pharmaceuticals; consultant for and research grant from Novo Nordisk; consultant for Reset Pharmaceuticals and Merck; expert witness for FTC.
Figures

References
-
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002; 165: 1217–1239. - PubMed
-
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000; 283: 1829–1836. - PubMed
-
- Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 2003; 290: 1906–1914. - PubMed
-
- Tuomilehto H, Seppa J, Uusitupa M. Obesity and obstructive sleep apnea - clinical significance of weight loss. Sleep Med Rev 2012; 17: 321–329. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

