Perfringolysin O Theta Toxin as a Tool to Monitor the Distribution and Inhomogeneity of Cholesterol in Cellular Membranes
- PMID: 27005662
- PMCID: PMC4810212
- DOI: 10.3390/toxins8030067
Perfringolysin O Theta Toxin as a Tool to Monitor the Distribution and Inhomogeneity of Cholesterol in Cellular Membranes
Abstract
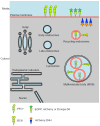
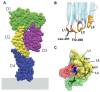
Cholesterol is an essential structural component of cellular membranes in eukaryotes. Cholesterol in the exofacial leaflet of the plasma membrane is thought to form membrane nanodomains with sphingolipids and specific proteins. Additionally, cholesterol is found in the intracellular membranes of endosomes and has crucial functions in membrane trafficking. Furthermore, cellular cholesterol homeostasis and regulation of de novo synthesis rely on transport via both vesicular and non-vesicular pathways. Thus, the ability to visualize and detect intracellular cholesterol, especially in the plasma membrane, is critical to understanding the complex biology associated with cholesterol and the nanodomains. Perfringolysin O (PFO) theta toxin is one of the toxins secreted by the anaerobic bacteria Clostridium perfringens and this toxin forms pores in the plasma membrane that causes cell lysis. It is well understood that PFO recognizes and binds to cholesterol in the exofacial leaflets of the plasma membrane, and domain 4 of PFO (D4) is sufficient for the binding of cholesterol. Recent studies have taken advantage of this high-affinity cholesterol-binding domain to create a variety of cholesterol biosensors by using a non-toxic PFO or the D4 in isolation. This review highlights the characteristics and usefulness of, and the principal findings related to, these PFO-derived cholesterol biosensors.
Keywords: biosensor; cholesterol; membranes; microscopy; perfringolysin O.
Figures

Similar articles
-
Domain 4 (D4) of Perfringolysin O to Visualize Cholesterol in Cellular Membranes-The Update.Sensors (Basel). 2017 Mar 3;17(3):504. doi: 10.3390/s17030504. Sensors (Basel). 2017. PMID: 28273804 Free PMC article. Review.
-
Perfringolysin O: The Underrated Clostridium perfringens Toxin?Toxins (Basel). 2015 May 14;7(5):1702-21. doi: 10.3390/toxins7051702. Toxins (Basel). 2015. PMID: 26008232 Free PMC article. Review.
-
R468A mutation in perfringolysin O destabilizes toxin structure and induces membrane fusion.Biochim Biophys Acta Biomembr. 2017 Jun;1859(6):1075-1088. doi: 10.1016/j.bbamem.2017.03.001. Epub 2017 Mar 2. Biochim Biophys Acta Biomembr. 2017. PMID: 28263714
-
Modifications in perfringolysin O domain 4 alter the cholesterol concentration threshold required for binding.Biochemistry. 2012 Apr 24;51(16):3373-82. doi: 10.1021/bi3003132. Epub 2012 Apr 13. Biochemistry. 2012. PMID: 22482748
-
Crucial role of perfringolysin O D1 domain in orchestrating structural transitions leading to membrane-perforating pores: a hydrogen-deuterium exchange study.J Biol Chem. 2014 Oct 10;289(41):28738-52. doi: 10.1074/jbc.M114.577981. Epub 2014 Aug 27. J Biol Chem. 2014. PMID: 25164812 Free PMC article.
Cited by
-
A Genome-Wide Screen of Deletion Mutants in the Filamentous Saccharomyces cerevisiae Background Identifies Ergosterol as a Direct Trigger of Macrophage Pyroptosis.mBio. 2018 Jul 31;9(4):e01204-18. doi: 10.1128/mBio.01204-18. mBio. 2018. PMID: 30065091 Free PMC article.
-
Measuring and Manipulating Membrane Cholesterol for the Study of Hedgehog Signaling.Methods Mol Biol. 2022;2374:73-87. doi: 10.1007/978-1-0716-1701-4_7. Methods Mol Biol. 2022. PMID: 34562244 Free PMC article.
-
Two distinct lipid transporters together regulate invasive filamentous growth in the human fungal pathogen Candida albicans.PLoS Genet. 2022 Dec 14;18(12):e1010549. doi: 10.1371/journal.pgen.1010549. eCollection 2022 Dec. PLoS Genet. 2022. PMID: 36516161 Free PMC article.
-
Mammalian membrane trafficking as seen through the lens of bacterial toxins.Cell Microbiol. 2020 Apr;22(4):e13167. doi: 10.1111/cmi.13167. Cell Microbiol. 2020. PMID: 32185902 Free PMC article. Review.
-
Plasma Membrane Origin of the Steroidogenic Pool of Cholesterol Used in Hormone-induced Acute Steroid Formation in Leydig Cells.J Biol Chem. 2016 Dec 9;291(50):26109-26125. doi: 10.1074/jbc.M116.740928. Epub 2016 Nov 3. J Biol Chem. 2016. PMID: 27815506 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

