TEX101, a glycoprotein essential for sperm fertility, is required for stable expression of Ly6k on testicular germ cells
- PMID: 27005865
- PMCID: PMC4804279
- DOI: 10.1038/srep23616
TEX101, a glycoprotein essential for sperm fertility, is required for stable expression of Ly6k on testicular germ cells
Abstract
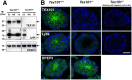
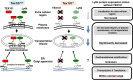
TEX101, a germ cell-specific glycosyl-phosphatidylinositol (GPI)-anchored glycoprotein, is associated with Ly6k during spermatogenesis in testis. Although both Tex101(-/-) and Ly6k(-/-) mice can produce morphologically intact spermatozoa, both knockout mice show an infertile phenotype due to a disorder of spermatozoa to migrate into the oviduct. Since Ly6k specifically interacts with TEX101, complex formation of TEX101/Ly6k appears to be potentially important for functional sperm production. This study evaluated the fate of Ly6k in the presence or absence of TEX101 to explore the molecular interaction of both GPI-anchored proteins in seminiferous tubules. The present study showed that: 1) Although Ly6k mRNA was detected, the protein was present at very low levels in mature testes of Tex101(-/-) mice, 2) Ly6k mRNA level was within the normal range in Tex101(-/-) mice, 3) Ly6k mRNA was translated into a polypeptide in the testes of Tex101(+/+) and Tex101(-/-) mice, and 4) TEX101, as well as Ly6k, are co-factors that affect to molecular expression. These results indicate that both TEX101 and Ly6k contribute to the post-translational counterpart protein expression at the cell membrane. This mechanism may be important in maintaining the production of fertile spermatozoa during spermatogenesis.
Figures



References
-
- Russell L. D. et al. Mammalian spermatogenesis in Histological and histopathological evaluation of the testis (ed. Russell L. D. et al.) 1–40 (Cache River Press, 1990).
-
- Bowles J. & Koopman P. Sex determination in mammalian germ cells: extrinsic versus intrinsic factors. Reproduction 139, 943–958 (2010). - PubMed
-
- Kurita A. et al. Identification, cloning, and initial characterization of a novel mouse testicular germ cell-specific antigen. Biol Reprod 64, 935–945 (2001). - PubMed
-
- Takayama T. et al. Sexually dimorphic expression of the novel germ cell antigen TEX101 during mouse gonad development. Biol Reprod 72, 1315–1323 (2005). - PubMed
-
- Jin H. et al. Molecular characterization of a germ-cell-specific antigen, TEX101, from mouse testis. Zygote 14, 201–208 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

