Long-term time-lapse live imaging reveals extensive cell migration during annelid regeneration
- PMID: 27006129
- PMCID: PMC4804569
- DOI: 10.1186/s12861-016-0104-2
Long-term time-lapse live imaging reveals extensive cell migration during annelid regeneration
Abstract
Background: Time-lapse imaging has proven highly valuable for studying development, yielding data of much finer resolution than traditional "still-shot" studies and allowing direct examination of tissue and cell dynamics. A major challenge for time-lapse imaging of animals is keeping specimens immobile yet healthy for extended periods of time. Although this is often feasible for embryos, the difficulty of immobilizing typically motile juvenile and adult stages remains a persistent obstacle to time-lapse imaging of post-embryonic development.
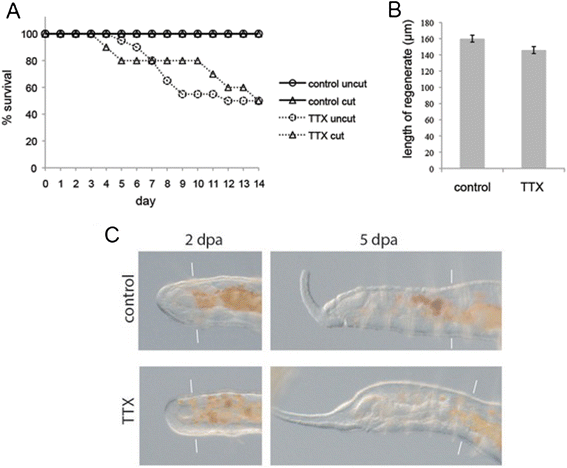
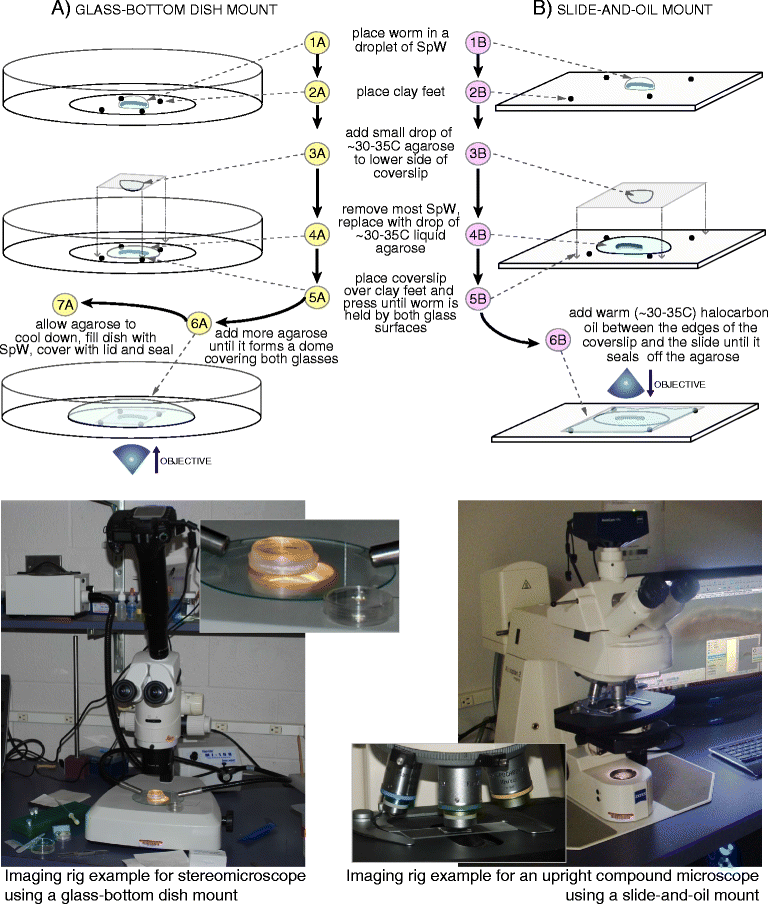
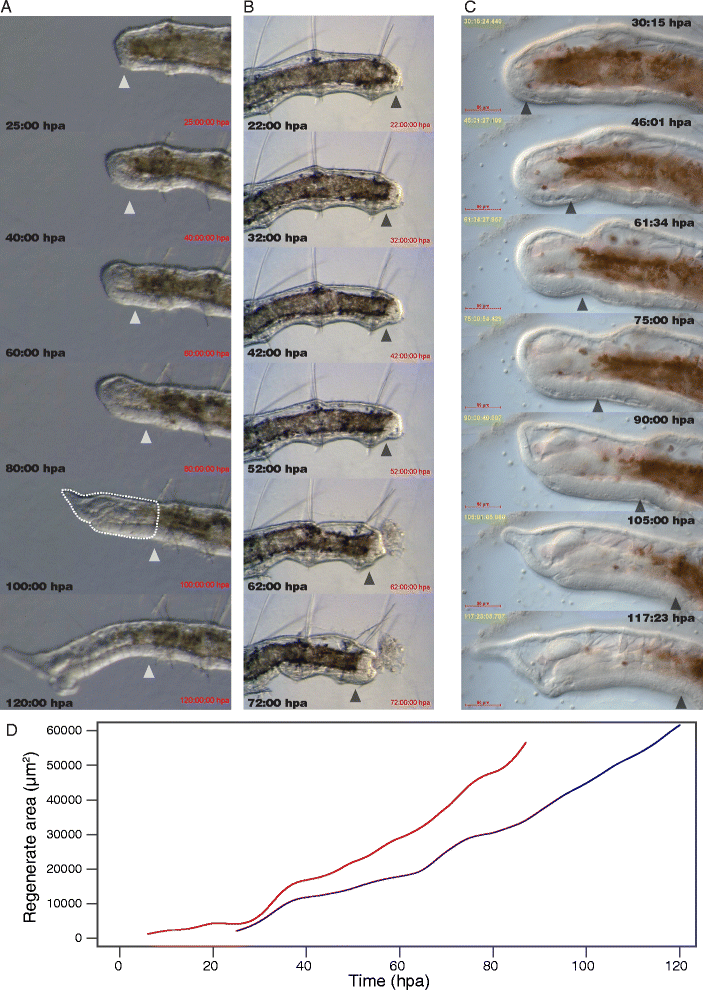
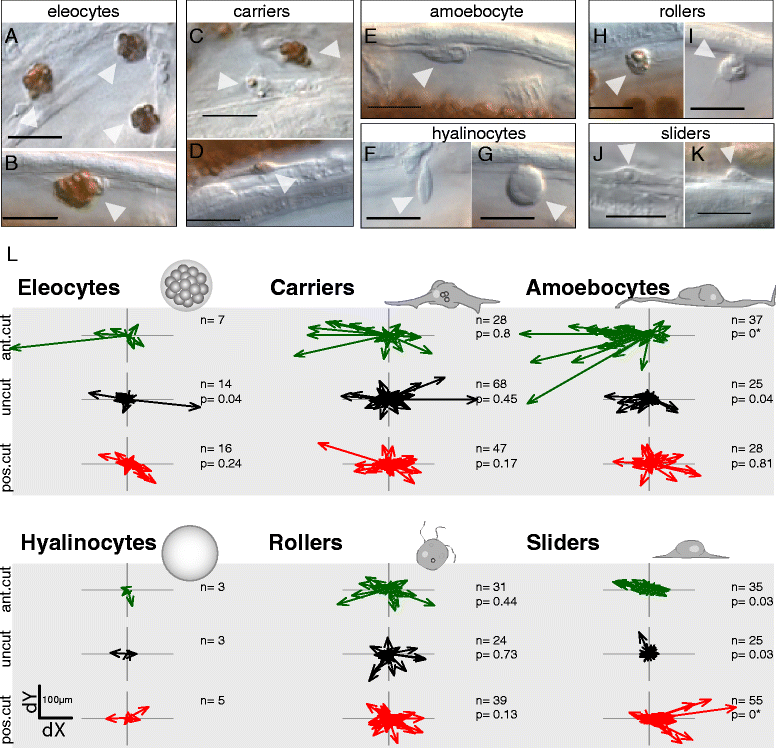
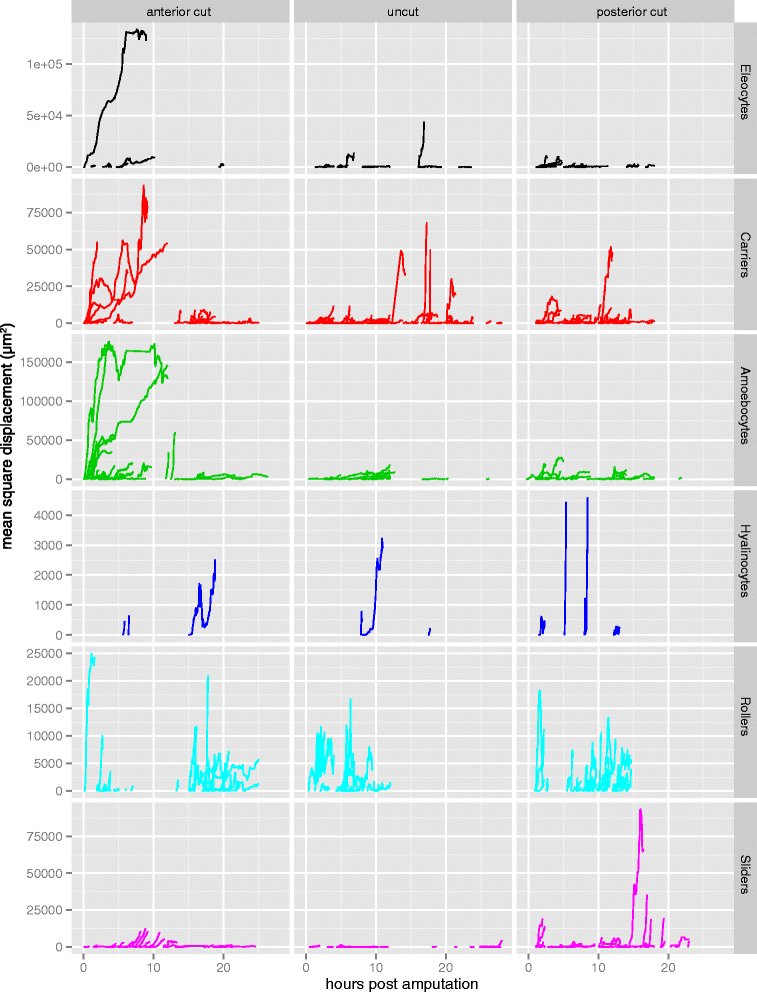
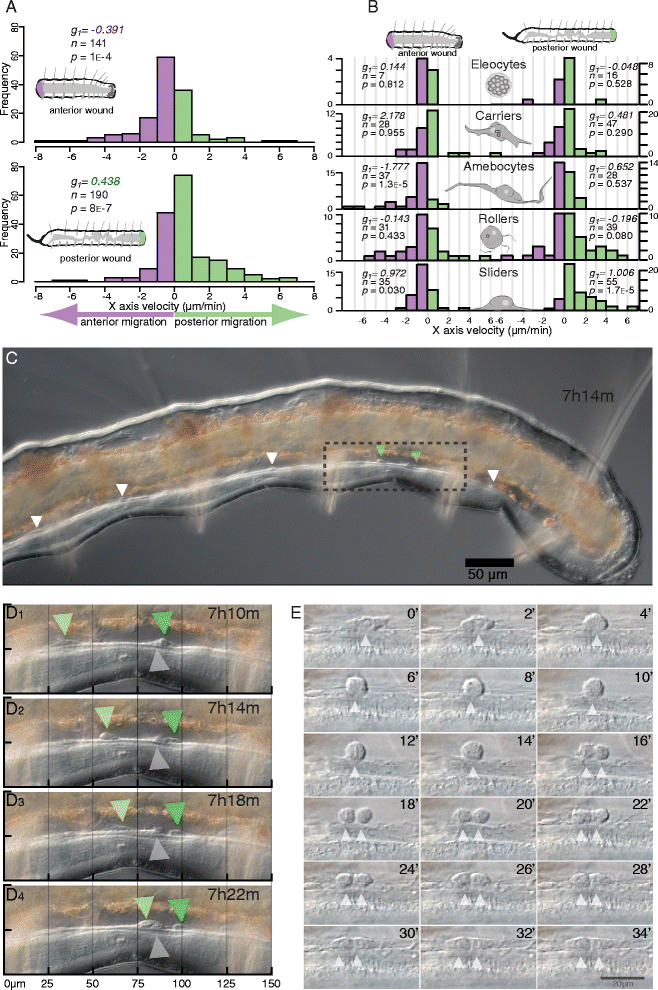
Results: Here we describe a new method for long-duration time-lapse imaging of adults of the small freshwater annelid Pristina leidyi and use this method to investigate its regenerative processes. Specimens are immobilized with tetrodotoxin, resulting in irreversible paralysis yet apparently normal regeneration, and mounted in agarose surrounded by culture water or halocarbon oil, to prevent dehydration but allowing gas exchange. Using this method, worms can be imaged continuously and at high spatial-temporal resolution for up to 5 days, spanning the entire regeneration process. We performed a fine-scale analysis of regeneration growth rate and characterized cell migration dynamics during early regeneration. Our studies reveal the migration of several putative cell types, including one strongly resembling published descriptions of annelid neoblasts, a cell type suggested to be migratory based on "still-shot" studies and long hypothesized to be linked to regenerative success in annelids.
Conclusions: Combining neurotoxin-based paralysis, live mounting techniques and a starvation-tolerant study system has allowed us to obtain the most extensive high-resolution longitudinal recordings of full anterior and posterior regeneration in an invertebrate, and to detect and characterize several cell types undergoing extensive migration during this process. We expect the tetrodotoxin paralysis and time-lapse imaging methods presented here to be broadly useful in studying other animals and of particular value for studying post-embryonic development.
Keywords: Annelid neoblast; Cell migration; Developmental dynamics; Growth rates; In-vivo studies; Regeneration; Time-lapse imaging.
Figures

References
-
- Boot MJ, Westerberg CH, Sanz-Ezquerro J, Cotterell J, Schweitzer R, Torres M, Sharpe J. In vitro whole-organ imaging: 4D quantification of growing mouse limb buds. Nat Meth. 2008;5:609–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

