Upfront bevacizumab may extend survival for glioblastoma patients who do not receive second-line therapy: an exploratory analysis of AVAglio
- PMID: 27006178
- PMCID: PMC4999000
- DOI: 10.1093/neuonc/now046
Upfront bevacizumab may extend survival for glioblastoma patients who do not receive second-line therapy: an exploratory analysis of AVAglio
Abstract
Background: In this post-hoc, exploratory analysis, we examined outcomes for patients enrolled in the AVAglio trial of front-line bevacizumab or placebo plus radiotherapy/temozolomide who received only a single line of therapy.
Methods: Patients with newly diagnosed glioblastoma received protocol-defined treatment until progressive disease (PD). Co-primary endpoints were investigator-assessed progression-free survival (PFS) and overall survival (OS). After confirmed PD, patients were treated at the investigators' discretion. PFS/OS were assessed in patients with a PFS event who did not receive post-PD therapy (Group 1) and patients with a PFS event who received post-PD therapy plus patients who did not have a PFS event at the final data cutoff (Group 2). Kaplan-Meier methodology was used. A multivariate Cox proportional hazards model for known prognostic variables was generated.
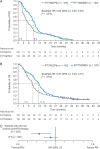
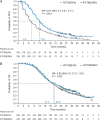
Results: Baseline characteristics were balanced. In patients with a PFS event who did not receive post-PD therapy (Group 1; n = 225 [24.4% of the intent-to-treat population]), the addition of bevacizumab to radiotherapy/temozolomide resulted in a 3.6-month extension in both median PFS (hazard ratio [HR]: 0.62, P = .0016) and median OS (HR: 0.67, P = .0102). Multivariate analyses supported this OS benefit (HR: 0.66). In the remaining patients (Group 2; n = 696), a 5.2-month PFS extension was observed in bevacizumab-treated patients (HR: 0.61, P < .0001); OS was comparable between the treatment arms (HR: 0.88, P = .1502). No significant differences in safety were observed between the 2 groups.
Conclusion: This exploratory analysis suggests that the addition of bevacizumab to standard glioblastoma treatment prolongs PFS and OS for patients with PD who receive only one line of therapy.
Trial registration: ClinicalTrials.gov NCT00943826.
Keywords: bevacizumab; crossover; glioblastoma; newly diagnosed; overall survival.
© The Author(s) 2016. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Stupp R, Hegi ME, Mason WP et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10 (5):459–466. - PubMed
-
- Chen JY, Hovey E, Rosenthal M et al. Neuro-oncology practices in Australia: a Cooperative Group for Neuro-Oncology patterns of care study. Asia Pac J Clin Oncol. 2014;10 (2):162–167. - PubMed
-
- Scoccianti S, Detti B, Cipressi S et al. Changes in neurocognitive functioning and quality of life in adult patients with brain tumors treated with radiotherapy. J Neurooncol. 2012;108 (2):291–308. - PubMed
-
- Hartmann C, Hentschel B, Simon M et al. Long-term survival in primary glioblastoma with versus without isocitrate dehydrogenase mutations. Clin Cancer Res. 2013;19 (18):5146–5157. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

