Gene expression profiling in undifferentiated thyroid carcinoma induced by high-dose radiation
- PMID: 27006382
- PMCID: PMC4915541
- DOI: 10.1093/jrr/rrw002
Gene expression profiling in undifferentiated thyroid carcinoma induced by high-dose radiation
Abstract
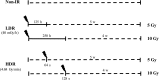
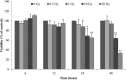
Published gene expression studies for radiation-induced thyroid carcinogenesis have used various methodologies. In this study, we identified differential gene expression in a human thyroid epithelial cell line after exposure to high-dose γ-radiation. HTori-3 cells were exposed to 5 or 10 Gy of ionizing radiation using two dose rates (high-dose rate: 4.68 Gy/min, and low-dose rate: 40 mGy/h) and then implanted into the backs of BALB/c nude mice after 4 (10 Gy) or 5 weeks (5 Gy). Decreases in cell viability, increases in giant cell frequency, anchorage-independent growth in vitro, and tumorigenicity in vivo were observed. Particularly, the cells irradiated with 5 Gy at the high-dose rate or 10 Gy at the low-dose rate demonstrated more prominent tumorigenicity. Gene expression profiling was analyzed via microarray. Numerous genes that were significantly altered by a fold-change of >50% following irradiation were identified in each group. Gene expression analysis identified six commonly misregulated genes, including CRYAB, IL-18, ZNF845, CYP24A1, OR4N4 and VN1R4, at all doses. These genes involve apoptosis, the immune response, regulation of transcription, and receptor signaling pathways. Overall, the altered genes in high-dose rate (HDR) 5 Gy and low-dose rate (LDR) 10 Gy were more than those of LDR 5 Gy and HDR 10 Gy. Thus, we investigated genes associated with aggressive tumor development using the two dosage treatments. In this study, the identified gene expression profiles reflect the molecular response following high doses of external radiation exposure and may provide helpful information about radiation-induced thyroid tumors in the high-dose range.
Keywords: carcinogenesis; cell line; gene expression; radiation; thyroid gland.
© The Author 2016. Published by Oxford University Press on behalf of The Japan Radiation Research Society and Japanese Society for Radiation Oncology.
Figures





Similar articles
-
Effect of subsequent acute-dose irradiation on cell survival in vitro following low dose-rate exposures.Int J Radiat Biol. 2002 Nov;78(11):981-90. doi: 10.1080/0955300021006589. Int J Radiat Biol. 2002. PMID: 12456285
-
Effect of dose rate on residual γ-H2AX levels and frequency of micronuclei in X-irradiated mouse lymphocytes.Radiat Res. 2015 Mar;183(3):315-24. doi: 10.1667/RR13860.1. Epub 2015 Mar 4. Radiat Res. 2015. PMID: 25738897 Free PMC article.
-
Transcriptional changes in U343 MG-a glioblastoma cell line exposed to ionizing radiation.Hum Exp Toxicol. 2008 Dec;27(12):919-29. doi: 10.1177/0960327108102045. Hum Exp Toxicol. 2008. PMID: 19273547
-
Overview of radiosensitivity of human tumor cells to low-dose-rate irradiation.Int J Radiat Oncol Biol Phys. 2008 Nov 1;72(3):909-17. doi: 10.1016/j.ijrobp.2008.06.1928. Int J Radiat Oncol Biol Phys. 2008. PMID: 19014780 Review.
-
Cellular responses and gene expression profiles of colonic Lgr5+ stem cells after low-dose/low-dose-rate radiation exposure.J Radiat Res. 2018 Apr 1;59(suppl_2):ii18-ii22. doi: 10.1093/jrr/rrx078. J Radiat Res. 2018. PMID: 29281035 Free PMC article. Review.
Cited by
-
Progression of the role of CRYAB in signaling pathways and cancers.Onco Targets Ther. 2019 May 30;12:4129-4139. doi: 10.2147/OTT.S201799. eCollection 2019. Onco Targets Ther. 2019. PMID: 31239701 Free PMC article. Review.
-
Differential expression of NPM, GSTA3, and GNMT in mouse liver following long-term in vivo irradiation by means of uranium tailings.Biosci Rep. 2018 Oct 17;38(5):BSR20180536. doi: 10.1042/BSR20180536. Print 2018 Oct 31. Biosci Rep. 2018. PMID: 30061177 Free PMC article.
-
Role of oxidative stress in Retinitis pigmentosa: new involved pathways by an RNA-Seq analysis.Cell Cycle. 2019 Jan;18(1):84-104. doi: 10.1080/15384101.2018.1558873. Epub 2018 Dec 28. Cell Cycle. 2019. Retraction in: Cell Cycle. 2020 Jan;19(2):256. doi: 10.1080/15384101.2019.1702311. PMID: 30569795 Free PMC article. Retracted.
-
Bcl2l10 induces metabolic alterations in ovarian cancer cells by regulating the TCA cycle enzymes SDHD and IDH1.Oncol Rep. 2021 Apr;45(4):47. doi: 10.3892/or.2021.7998. Epub 2021 Mar 2. Oncol Rep. 2021. PMID: 33649794 Free PMC article.
-
Effect of low-dose radiation on thyroid function and the gut microbiota.World J Gastroenterol. 2022 Oct 14;28(38):5557-5572. doi: 10.3748/wjg.v28.i38.5557. World J Gastroenterol. 2022. PMID: 36304083 Free PMC article.
References
-
- Sassolas G, Hafdi-Nejjari Z, Casagranda L et al. . Thyroid cancers in children, adolescents, and young adults with and without a history of childhood exposure to therapeutic radiation for other cancers. Thyroid 2013;23:805–10. - PubMed
-
- Hamatani K, Eguchi H, Ito R et al. . RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res 2008;68:7176–82. - PubMed
-
- Schlumberger M, Le Guen B. Nuclear-power-plant accidents: thyroid cancer incidence and radiation-related health effects from the Chernobyl accident. Med Sci (Paris) 2012;28:746–56. - PubMed
-
- Schonfeld SJ, Lee C, Berrington deGonzález A. Medical exposure to radiation and thyroid cancer. Clin Oncol (R Coll Radiol) 2011;23:244–50. - PubMed
-
- Preston DL, Ron E, Tokuoka S et al. . Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 2007;168:1–64. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

