Brain-derived Neurotrophic Factor in Megakaryocytes
- PMID: 27006395
- PMCID: PMC4858990
- DOI: 10.1074/jbc.M116.720029
Brain-derived Neurotrophic Factor in Megakaryocytes
Abstract
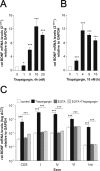
The biosynthesis of endogenous brain-derived neurotrophic factor (BDNF) has thus far been examined in neurons where it is expressed at very low levels, in an activity-dependent fashion. In humans, BDNF has long been known to accumulate in circulating platelets, at levels far higher than in the brain. During the process of blood coagulation, BDNF is released from platelets, which has led to its extensive use as a readily accessible biomarker, under the assumption that serum levels may somehow reflect brain levels. To identify the cellular origin of BDNF in platelets, we established primary cultures of megakaryocytes, the progenitors of platelets, and we found that human and rat megakaryocytes express the BDNF gene. Surprisingly, the pattern of mRNA transcripts is similar to neurons. In the presence of thapsigargin and external calcium, the levels of the mRNA species leading to efficient BDNF translation rapidly increase. Under these conditions, pro-BDNF, the obligatory precursor of biologically active BDNF, becomes readily detectable. Megakaryocytes store BDNF in α-granules, with more than 80% of them also containing platelet factor 4. By contrast, BDNF is undetectable in mouse megakaryocytes, in line with the absence of BDNF in mouse serum. These findings suggest that alterations of BDNF levels in human serum as reported in studies dealing with depression or physical exercise may primarily reflect changes occurring in megakaryocytes and platelets, including the ability of the latter to retain and release BDNF.
Keywords: blood; brain-derived neurotrophic factor (BDNF); neurobiology; neurochemistry; neurotrophin.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Park H., and Poo M. M. (2013) Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 14, 7–23 - PubMed
-
- Zagrebelsky M., and Korte M. (2014) Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology 76, 628–638 - PubMed
-
- Egan M. F., Kojima M., Callicott J. H., Goldberg T. E., Kolachana B. S., Bertolino A., Zaitsev E., Gold B., Goldman D., Dean M., Lu B., and Weinberger D. R. (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269 - PubMed
-
- Gray J., Yeo G. S., Cox J. J., Morton J., Adlam A. L., Keogh J. M., Yanovski J. A., El Gharbawy A., Han J. C., Tung Y. C., Hodges J. R., Raymond F. L., O'rahilly S., and Farooqi I. S. (2006) Hyperphagia, severe obesity, impaired cognitive function, and hyperactivity associated with functional loss of one copy of the brain-derived neurotrophic factor (BDNF) gene. Diabetes 55, 3366–3371 - PMC - PubMed
-
- Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., and Barde Y. A. (1989) Molecular cloning and expression of brain-derived neurotrophic factor. Nature 341, 149–152 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

