Crystal structure of bis-(2-methyl-1H-imidazol-3-ium) di-hydroxidobis(oxalato-κ(2) O (1),O (2))stannate(IV) monohydrate
- PMID: 27006807
- PMCID: PMC4778813
- DOI: 10.1107/S2056989016002061
Crystal structure of bis-(2-methyl-1H-imidazol-3-ium) di-hydroxidobis(oxalato-κ(2) O (1),O (2))stannate(IV) monohydrate
Abstract
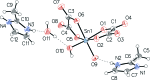
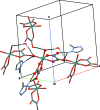
In the structure of the hydrated title salt, (C4H7N2)2[Sn(C2O4)2(OH)2]·H2O, the asymmetric unit comprises one stannate(IV) dianion, two organic cations and one water mol-ecule of crystallization. The [Sn(C2O4)2(OH)2](2-) dianion consists of an Sn(IV) atom chelated by two oxalate anions and coordinated by two OH(-) ligands in a cis octa-hedral arrangement. Neighbouring anions are connected through O-H⋯O hydrogen bonds between hydroxide groups and non-coordinating oxalate O atoms into layers expanding parallel to (100). In addition, cations and anions are linked through N-H⋯O hydrogen bonds, and the water mol-ecule bridges two anions with two O-H⋯O hydrogen bonds and is also the acceptor of an N-H⋯O hydrogen bond with one of the cations. Weak C-H⋯O hydrogen bonds are also observed. The intricate hydrogen bonding leads to the formation of a three-dimensional network.
Keywords: crystal structure; hydrogen bonds; organotin(IV) complex.
Figures
References
-
- Bruker (2014). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Davies, A. G., Gielen, M., Pannell, K. H. & Tiekink, E. R. T. (2008). In Tin Chemistry, Fundamentals, Frontiers and Applications. Chichester, UK: John Wiley & Sons Ltd.
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
