Injected nanocrystals for targeted drug delivery
- PMID: 27006893
- PMCID: PMC4788714
- DOI: 10.1016/j.apsb.2015.11.005
Injected nanocrystals for targeted drug delivery
Abstract
Nanocrystals are pure drug crystals with sizes in the nanometer range. Due to the advantages of high drug loading, platform stability, and ease of scaling-up, nanocrystals have been widely used to deliver poorly water-soluble drugs. Nanocrystals in the blood stream can be recognized and sequestered as exogenous materials by mononuclear phagocytic system (MPS) cells, leading to passive accumulation in MPS-rich organs, such as liver, spleen and lung. Particle size, morphology and surface modification affect the biodistribution of nanocrystals. Ligand conjugation and stimuli-responsive polymers can also be used to target nanocrystals to specific pathogenic sites. In this review, the progress on injected nanocrystals for targeted drug delivery is discussed following a brief introduction to nanocrystal preparation methods, i.e., top-down and bottom-up technologies.
Keywords: Biodistribution; Encapsulation; Ligand; Nanocrystals; Stimuli response; Targeted drug delivery.
Figures

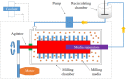
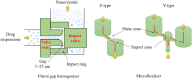

References
-
- Ranjita S. Nanosuspensions: a new approach for organ and cellular targeting in infectious diseases. J Pharm Invest. 2013;43:1–26.
-
- Trapani G., Denora N., Trapani A., Laquintana V. Recent advances in ligand targeted therapy. J Drug Target. 2012;20:1–22. - PubMed
-
- Hollis C.P., Zhao R., Li T. Hybrid nanocrystal as a versatile platform for cancer theranostics. In: Park K., editor. Biomaterials for cancer therapeutics: diagnosis, prevention and therapy. Cambridge: Woodhead Publishing; 2013. pp. 186–204.
-
- Lu Y., Chen Y., Gemeinhart R.A., Wu W., Li T. Developing nanocrystals for cancer treatment. Nanomedicine (Lond) 2015;10:2537–2552. - PubMed
-
- Keck C.M., Muller R.H. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur J Pharm Biopharm. 2006;62:3–16. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

