Tamoxifen Promotes Axonal Preservation and Gait Locomotion Recovery after Spinal Cord Injury in Cats
- PMID: 27006979
- PMCID: PMC4781988
- DOI: 10.1155/2016/9561968
Tamoxifen Promotes Axonal Preservation and Gait Locomotion Recovery after Spinal Cord Injury in Cats
Abstract
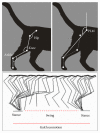
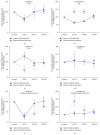
We performed experiments in cats with a spinal cord penetrating hemisection at T13-L1 level, with and without tamoxifen treatment. The results showed that the numbers of the ipsilateral and contralateral ventral horn neurons were reduced to less than half in the nontreated animals compared with the treated ones. Also, axons myelin sheet was preserved to almost normal values in treated cats. On the contrary, in the untreated animals, their myelin sheet was reduced to 28% at 30 days after injury (DAI), in both the ipsilateral and contralateral regions of the spinal cord. Additionally, we made hindlimb kinematics experiments to study the effects of tamoxifen on cat locomotion after the injury: at 4, 16, and 30 DAI. We observed that the ipsilateral hindlimb angular displacement (AD) of the pendulum-like movements (PLM) during gait locomotion was recovered to almost normal values in treated cats. Contralateral PLM acquired similar values to those obtained in intact cats. At 4 DAI, untreated animals showed a compensatory increment of PLM occurring in the contralateral hindlimb, which was partially recovered at 30 DAI. Our findings indicate that tamoxifen exerts a neuroprotective effect and preserves or produces myelinated axons, which could benefit the locomotion recovery in injured cats.
Figures






Similar articles
-
Quantitative analysis of hindlimbs locomotion kinematics in spinalized rats treated with Tamoxifen plus treadmill exercise.Neuroscience. 2016 Oct 1;333:151-61. doi: 10.1016/j.neuroscience.2016.07.023. Epub 2016 Jul 20. Neuroscience. 2016. PMID: 27450566
-
Recovery of hindlimb locomotion after incomplete spinal cord injury in the cat involves spontaneous compensatory changes within the spinal locomotor circuitry.J Neurophysiol. 2011 Oct;106(4):1969-84. doi: 10.1152/jn.00368.2011. Epub 2011 Jul 20. J Neurophysiol. 2011. PMID: 21775717
-
Thoracic Hemisection in Rats Results in Initial Recovery Followed by a Late Decrement in Locomotor Movements, with Changes in Coordination Correlated with Serotonergic Innervation of the Ventral Horn.PLoS One. 2015 Nov 25;10(11):e0143602. doi: 10.1371/journal.pone.0143602. eCollection 2015. PLoS One. 2015. PMID: 26606275 Free PMC article.
-
The "beneficial" effects of locomotor training after various types of spinal lesions in cats and rats.Prog Brain Res. 2015;218:173-98. doi: 10.1016/bs.pbr.2014.12.009. Epub 2015 Mar 29. Prog Brain Res. 2015. PMID: 25890137 Review.
-
Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury.Prog Brain Res. 2002;137:257-73. doi: 10.1016/s0079-6123(02)37020-1. Prog Brain Res. 2002. PMID: 12440372 Review.
Cited by
-
Continuous tamoxifen delivery improves locomotor recovery 6h after spinal cord injury by neuronal and glial mechanisms in male rats.Exp Neurol. 2018 Jan;299(Pt A):109-121. doi: 10.1016/j.expneurol.2017.10.006. Epub 2017 Oct 13. Exp Neurol. 2018. PMID: 29037533 Free PMC article.
-
Tamoxifen Provides Structural and Functional Rescue in Murine Models of Photoreceptor Degeneration.J Neurosci. 2017 Mar 22;37(12):3294-3310. doi: 10.1523/JNEUROSCI.2717-16.2017. Epub 2017 Feb 24. J Neurosci. 2017. PMID: 28235894 Free PMC article.
-
Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery.Neural Regen Res. 2016 Aug;11(8):1208-11. doi: 10.4103/1673-5374.189164. Neural Regen Res. 2016. PMID: 27651756 Free PMC article. Review.
-
Characteristics of adverse events of endocrine therapies among older patients with breast cancer.Support Care Cancer. 2019 Oct;27(10):3813-3822. doi: 10.1007/s00520-019-04674-8. Epub 2019 Feb 7. Support Care Cancer. 2019. PMID: 30729298
-
Effects of Dietary Vitamin E Supplementation in Bladder Function and Spasticity during Spinal Cord Injury.Brain Sci. 2018 Feb 26;8(3):38. doi: 10.3390/brainsci8030038. Brain Sci. 2018. PMID: 29495419 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

