Transcription factor RUNX2 up-regulates chemokine receptor CXCR4 to promote invasive and metastatic potentials of human gastric cancer
- PMID: 27007162
- PMCID: PMC4991507
- DOI: 10.18632/oncotarget.8236
Transcription factor RUNX2 up-regulates chemokine receptor CXCR4 to promote invasive and metastatic potentials of human gastric cancer
Abstract
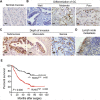
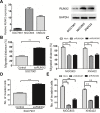
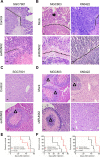
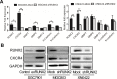
Runt-related transcription factor 2 (RUNX2) is a regulator of embryogenesis and development, but has also been implicated in the progression of certain human cancer. This study aimed to elucidate the role of RUNX2 in the invasive and metastatic potentials of human gastric cancer (GC) and the underlying mechanisms. We found that the levels of RUNX2 expression in gastric cancer tissues were correlated with the differentiation degrees, invasion depth and lymph node metastasis. COX regression analysis indicated that RUNX2 was an independent prognostic indicator for GC patients. RUNX2 significantly increased the migration and invasion ability of GC cells in vitro and enhanced the invasion and metastatic potential of GC cells in an orthotopic GC model of nude mice. Mechanistically, RUNX2 directly bound to the promoter region of the gene coding for the chemokine receptor CXCR4 to enhance its transcription. CXCR4 knockdown or treatment with AMD3100, a CXCR4 inhibitor, attenuated RUNX2-promoted invasion and metastasis. These results demonstrate that RUNX2 promotes the invasion and metastasis of human GC by transcriptionally up-regulating the chemokine receptor CXCR4. Therefore, the RUNX2-CXCR4 axis is a potential therapeutic target for GC.
Keywords: CXCR4; RUNX2; gastric cancer; invasion; metastasis.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
A positive crosstalk between CXCR4 and CXCR2 promotes gastric cancer metastasis.Oncogene. 2017 Sep 7;36(36):5122-5133. doi: 10.1038/onc.2017.108. Epub 2017 May 8. Oncogene. 2017. PMID: 28481874
-
The transcription factor RUNX2 fuels YAP1 signaling and gastric cancer tumorigenesis.Cancer Sci. 2021 Sep;112(9):3533-3544. doi: 10.1111/cas.15045. Epub 2021 Jul 16. Cancer Sci. 2021. PMID: 34160112 Free PMC article.
-
LncRNA COL1A1-014 is involved in the progression of gastric cancer via regulating CXCL12-CXCR4 axis.Gastric Cancer. 2020 Mar;23(2):260-272. doi: 10.1007/s10120-019-01011-0. Epub 2019 Oct 24. Gastric Cancer. 2020. PMID: 31650323
-
Oncostatin M receptor, positively regulated by SP1, promotes gastric cancer growth and metastasis upon treatment with Oncostatin M.Gastric Cancer. 2019 Sep;22(5):955-966. doi: 10.1007/s10120-019-00934-y. Epub 2019 Feb 18. Gastric Cancer. 2019. PMID: 30778797
-
Chemokines and chemokine receptors: new insights into cancer-related inflammation.Trends Mol Med. 2010 Mar;16(3):133-44. doi: 10.1016/j.molmed.2010.01.003. Epub 2010 Feb 15. Trends Mol Med. 2010. PMID: 20163989 Free PMC article. Review.
Cited by
-
RUNX2 overexpression and PTEN haploinsufficiency cooperate to promote CXCR7 expression and cellular trafficking, AKT hyperactivation and prostate tumorigenesis.Theranostics. 2019 May 24;9(12):3459-3475. doi: 10.7150/thno.33292. eCollection 2019. Theranostics. 2019. PMID: 31281490 Free PMC article.
-
WWOX inhibits the invasion of lung cancer cells by downregulating RUNX2.Cancer Gene Ther. 2016 Dec;23(12):433-438. doi: 10.1038/cgt.2016.59. Epub 2016 Nov 11. Cancer Gene Ther. 2016. PMID: 27834355
-
Inhibition of CXCL12/CXCR4 axis as a potential targeted therapy of advanced gastric carcinoma.Cancer Med. 2017 Jun;6(6):1424-1436. doi: 10.1002/cam4.1085. Epub 2017 May 23. Cancer Med. 2017. PMID: 28544785 Free PMC article. Review.
-
Runx2 is required for activity of CD44+/CD24-/low breast cancer stem cell in breast cancer development.Am J Transl Res. 2020 May 15;12(5):2305-2318. eCollection 2020. Am J Transl Res. 2020. PMID: 32509221 Free PMC article.
-
PAX3 Promotes Cell Migration and CXCR4 Gene Expression in Neural Crest Cells.J Mol Neurosci. 2018 Jan;64(1):1-8. doi: 10.1007/s12031-017-0995-9. Epub 2017 Nov 13. J Mol Neurosci. 2018. PMID: 29134414
References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Onate-Ocana LF, Mendez-Cruz G, Hernandez-Ramos R, Becker M, Carrillo JF, Herrera-Goepfert R, Aiello-Crocifoglio V, Ochoa-Carrillo F, Beltran-Ortega A. Experience of surgical morbidity after palliative surgery in patients with gastric carcinoma. Gastric Cancer. 2007;10:215–220. - PubMed
-
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. - PubMed
-
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. - PubMed
-
- Pratap J, Galindo M, Zaidi SK, Vradii D, Bhat BM, Robinson JA, Choi JY, Komori T, Stein JL, Lian JB, Stein GS, van Wijnen AJ. Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res. 2003;63:5357–5362. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

