Antimicrobial dressings for the prevention of catheter-related infections in newborn infants with central venous catheters
- PMID: 27007217
- PMCID: PMC6464939
- DOI: 10.1002/14651858.CD011082.pub2
Antimicrobial dressings for the prevention of catheter-related infections in newborn infants with central venous catheters
Abstract
Background: Central venous catheters (CVCs) provide secured venous access in neonates. Antimicrobial dressings applied over the CVC sites have been proposed to reduce catheter-related blood stream infection (CRBSI) by decreasing colonisation. However, there may be concerns on the local and systemic adverse effects of these dressings in neonates.
Objectives: We assessed the effectiveness and safety of antimicrobial (antiseptic or antibiotic) dressings in reducing CVC-related infections in newborn infants. Had there been relevant data, we would have evaluated the effects of antimicrobial dressings in different subgroups, including infants who received different types of CVCs, infants who required CVC for different durations, infants with CVCs with and without other antimicrobial modifications, and infants who received an antimicrobial dressing with and without a clearly defined co-intervention.
Search methods: We used the standard search strategy of the Cochrane Neonatal Review Group (CNRG). We searched the Cochrane Central Register of Controlled Trials (The Cochrane Library 2015, Issue 9), MEDLINE (PubMed), EMBASE (EBCHOST), CINAHL and references cited in our short-listed articles using keywords and MeSH headings, up to September 2015.
Selection criteria: We included randomised controlled trials that compared an antimicrobial CVC dressing against no dressing or another dressing in newborn infants.
Data collection and analysis: We extracted data using the standard methods of the CNRG. Two review authors independently assessed the eligibility and risk of bias of the retrieved records. We expressed our results using risk difference (RD) and risk ratio (RR) with 95% confidence intervals (CIs).
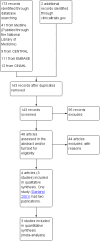
Main results: Out of 173 articles screened, three studies were included. There were two comparisons: chlorhexidine dressing following alcohol cleansing versus polyurethane dressing following povidone-iodine cleansing (one study); and silver-alginate patch versus control (two studies). A total of 855 infants from level III neonatal intensive care units (NICUs) were evaluated, 705 of whom were from a single study. All studies were at high risk of bias for blinding of care personnel or unclear risk of bias for blinding of outcome assessors. There was moderate-quality evidence for all major outcomes.The single study comparing chlorhexidine dressing/alcohol cleansing against polyurethane dressing/povidone-iodine cleansing showed no significant difference in the risk of CRBSI (RR 1.18, 95% CI 0.53 to 2.65; RD 0.01, 95% CI -0.02 to 0.03; 655 infants, moderate-quality evidence) and sepsis without a source (RR 1.06, 95% CI 0.75 to 1.52; RD 0.01, 95% CI -0.04 to 0.06; 705 infants, moderate-quality evidence). There was a significant reduction in the risk of catheter colonisation favouring chlorhexidine dressing/alcohol cleansing group (RR 0.62, 95% CI 0.45 to 0.86; RD -0.09, 95% CI -0.15 to -0.03; number needed to treat for an additional beneficial outcome (NNTB) 11, 95% CI 7 to 33; 655 infants, moderate-quality evidence). However, infants in the chlorhexidine dressing/alcohol cleansing group were significantly more likely to develop contact dermatitis, with 19 infants in the chlorhexidine dressing/alcohol cleansing group having developed contact dermatitis compared to none in the polyurethane dressing/povidone-iodine cleansing group (RR 43.06, 95% CI 2.61 to 710.44; RD 0.06, 95% CI 0.03 to 0.08; number needed to treat for an additional harmful outcome (NNTH) 17, 95% CI 13 to 33; 705 infants, moderate-quality evidence). The roles of chlorhexidine dressing in the outcomes reported were unclear, as the two assigned groups received different co-interventions in the form of different skin cleansing agents prior to catheter insertion and during each dressing change.In the other comparison, silver-alginate patch versus control, the data for CRBSI were analysed separately in two subgroups as the two included studies reported the outcome using different denominators: one using infants and another using catheters. There were no significant differences between infants who received silver-alginate patch against infants who received standard line dressing in CRBSI, whether expressed as the number of infants (RR 0.50, 95% CI 0.14 to 1.78; RD -0.12, 95% CI -0.33 to 0.09; 1 study, 50 participants, moderate-quality evidence) or as the number of catheters (RR 0.72, 95% CI 0.27 to 1.89; RD -0.05, 95% CI -0.20 to 0.10; 1 study, 118 participants, moderate-quality evidence). There was also no significant difference between the two groups in mortality (RR 0.55, 95% CI 0.15 to 2.05; RD -0.04, 95% CI -0.13 to 0.05; two studies, 150 infants, I² = 0%, moderate-quality evidence). No adverse skin reaction was recorded in either group.
Authors' conclusions: Based on moderate-quality evidence, chlorhexidine dressing/alcohol skin cleansing reduced catheter colonisation, but made no significant difference in major outcomes like sepsis and CRBSI compared to polyurethane dressing/povidone-iodine cleansing. Chlorhexidine dressing/alcohol cleansing posed a substantial risk of contact dermatitis in preterm infants, although it was unclear whether this was contributed mainly by the dressing material or the cleansing agent. While silver-alginate patch appeared safe, evidence is still insufficient for a recommendation in practice. Future research that evaluates antimicrobial dressing should ensure blinding of caregivers and outcome assessors and ensure that all participants receive the same co-interventions, such as the skin cleansing agent. Major outcomes like sepsis, CRBSI and mortality should be assessed in infants of different gestation and birth weight.
Conflict of interest statement
None declared by the authors
Figures

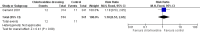

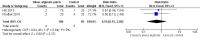








Update of
- doi: 10.1002/14651858.CD011082
References
References to studies included in this review
Garland 2001 {published data only}
-
- Garland JS, Alex CP, Mueller CD, Cisler‐Kahill LA. Local reactions to a chlorhexidine gluconate‐impregnated antimicrobial dressing in very low birth weight infants. Pediatric Infectious Disease Journal 1996;15(10):912‐4. - PubMed
-
- Garland JS, Alex CP, Mueller CD, Otten D, Shivpuri C, Harris MC, et al. A randomized trial comparing povidone‐iodine to a chlorhexidine gluconate‐impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics 2001;107(6):1431‐6. - PubMed
Hill 2010 {published data only}
-
- Hill ML, Baldwin L, Slaughter JC, Walsh WF, Weitkamp JH. A silver‐alginate‐coated dressing to reduce peripherally inserted central catheter (PICC) infections in NICU patients: a pilot randomized controlled trial. Journal of Perinatology 2010;30(7):469‐73. - PubMed
Khattak 2010 {published data only}
-
- Khattak AZ, Ross R, Ngo T, Shoemaker CT. A randomized controlled evaluation of absorption of silver with the use of silver alginate (Algidex) patches in very low birth weight (VLBW) infants with central lines. Journal of Perinatology 2010;30(5):337‐42. - PubMed
References to studies excluded from this review
Bertini 2013 {published data only}
-
- Bertini G, Elia S, Ceciarini F, Dani C. Reduction of catheter‐related bloodstream infections in preterm infants by the use of catheters with the AgION antimicrobial system. Early Human Development 2013;89(1):21‐5. - PubMed
Brito 2014 {published data only}
Brooker 2007 {published data only}
-
- Brooker RW, Keenan WJ. Catheter related bloodstream infection following PICC removal in preterm infants. Journal of Perinatology 2007;27(3):171‐4. - PubMed
Casner 2014 {published data only}
-
- Casner M, Hoesli SJ, Slaughter JC, Hill M, Weitkamp JH. Incidence of catheter‐related bloodstream infections in neonates following removal of peripherally inserted central venous catheters. Pediatric Critical Care Medicine 2014;15(1):42‐8. - PubMed
Cox 2013 {published data only}
-
- Cox EG, Knoderer CA, Jennings A, Brown JW, Rodefeld MD, Walker SG, et al. A randomized, controlled trial of catheter‐related infectious event rates using antibiotic‐impregnated catheters versus conventional catheters in pediatric cardiovascular surgery patients. Journal of the Pediatric Infectious Diseases Society 2013;2(1):67‐70. - PubMed
Dawson 2000 {published data only}
-
- Dawson S, Fitzgerald P, Langer JC, Walton M, Winthrop A, Lau G, et al. A preoperative protocol for the prevention of infection in children with tunnelled right atrial catheters. Oncology Reports 2000;7(6):1239‐42. - PubMed
Fallat 1998 {published data only}
-
- Fallat ME, Gallinaro RN, Stover BH, Wilkerson S, Goldsmith LJ. Central venous catheter bloodstream infections in the neonatal intensive care unit. Journal of Pediatric Surgery 1998;33(9):1383‐7. - PubMed
Fanos 1999 {published data only}
-
- Fanos V, Dall'Agnola A. Antibiotics in neonatal infections: a review. Drugs 1999;58(3):405‐27. - PubMed
Filippi 2007 {published data only}
-
- Filippi L, Pezzati M, Amario S, Poggi C, Pecile P. Fusidic acid and heparin lock solution for the prevention of catheter‐related bloodstream infections in critically ill neonates: a retrospective study and a prospective, randomized trial. Pediatric Critical Care Medicine 2007;8(6):556‐62. - PubMed
Garcia‐Teresa 2007 {published data only}
-
- Garcia‐Teresa MA, Casado‐Flores J, Delgado Dominguez M A, Roqueta‐Mas J, Cambra‐Lasaosa F, Concha‐Torre A, et al. Infectious complications of percutaneous central venous catheterization in pediatric patients: a Spanish multicenter study. Intensive Care Medicine 2007;33(3):466‐76. - PubMed
Garland 2005 {published data only}
-
- Garland JS, Alex CP, Henrickson KJ, McAuliffe TL, Maki DG. A vancomycin‐heparin lock solution for prevention of nosocomial bloodstream infection in critically ill neonates with peripherally inserted central venous catheters: a prospective, randomized trial. Pediatrics 2005;116(2):e198‐205. - PubMed
Garland 2008 {published data only}
-
- Garland JS, Alex CP, Sevallius JM, Murphy DM, Good MJ, Volberding AM, et al. Cohort study of the pathogenesis and molecular epidemiology of catheter‐related bloodstream infection in neonates with peripherally inserted central venous catheters. Infection Control and Hospital Epidemiology 2008;29(3):243‐9. - PubMed
Garland 2009 {published data only}
-
- Garland JS, Alex CP, Uhing MR, Peterside IE, Rentz A, Harris MC. Pilot trial to compare tolerance of chlorhexidine gluconate to povidone‐iodine antisepsis for central venous catheter placement in neonates. Journal of Perinatology 2009;29(12):808‐13. - PubMed
Gilad 2006 {published data only}
-
- Gilad J, Borer A. Prevention of catheter‐related bloodstream infections in the neonatal intensive care setting. Expert Review of Anti‐Infective Therapy 2006;4(5):861‐73. - PubMed
Gilbert 2008 {published data only}
-
- Gilbert RE, Harden M. Effectiveness of impregnated central venous catheters for catheter related blood stream infection: a systematic review. Current Opinion in Infectious Diseases 2008;21(3):235‐45. - PubMed
Golombek 2002 {published data only}
-
- Golombek SG, Rohan AJ, Parvez B, Salice AL, LaGamma EF. "Proactive" management of percutaneously inserted central catheters results in decreased incidence of infection in the ELBW population. Journal of Perinatology 2002;22(3):209‐13. - PubMed
Gravel 2007 {published data only}
-
- Gravel D, Matlow A, Ofner‐Agostini M, Loeb M, Johnston L, Bryce E, et al. A point prevalence survey of health care‐associated infections in pediatric populations in major Canadian acute care hospitals. American Journal of Infection Control 2007;35(3):157‐62. - PubMed
Grigor'ev 1993 {published data only}
-
- Grigor'ev VE, Pankova VP. Prevention of suppurative‐inflammatory complications in catheterization of central veins. Anesteziologiia i Reanimatologiia 1993;2(2):63‐4. - PubMed
Handrup 2012 {published data only}
-
- Handrup MM, Fuursted K, Funch P, Moller JK, Schroder H. Biofilm formation in long‐term central venous catheters in children with cancer: A randomized controlled open‐labelled trial of taurolidine versus heparin. Acta Pathologica, Microbiologica, et Immunologica Scandinavica 2012;120(10):794‐801. - PubMed
Harms 1995 {published data only}
-
- Harms K, Herting E, Kron M, Schiffmann H, Schulz‐Ehlbeck H. Randomized, controlled trial of amoxicillin prophylaxis for prevention of catheter‐related infections in newborn infants with central venous silicone elastomer catheters. Journal of Pediatrics 1995;127(4):615‐9. - PubMed
Hei 2012 {published data only}
-
- Hei MY, Zhang XC, Gao XY, Zhao LL, Wu ZX, Tian L, et al. Catheter‐related infection and pathogens of umbilical venous catheterization in a neonatal intensive care unit in China. American Journal of Perinatology 2012;29(2):107‐14. - PubMed
Helder 2013 {published data only}
-
- Helder O, Hoogen A, Boer C, Goudoever J, Verboon‐Maciolek M, Kornelisse R. Non pharmacological interventions to reduce bloodstream infections in infants admitted to a neonatal intensive care unit. Intensive Care Medicine 2013;39:S6. - PubMed
Hemels 2011 {published data only}
-
- Hemels MA, Hoogen A, Verboon‐Maciolek MA, Fleer A, Krediet TG. Prevention of neonatal late‐onset sepsis associated with the removal of percutaneously inserted central venous catheters in preterm infants. Pediatric Critical Care Medicine 2011;12(4):445‐8. - PubMed
Huang 2011 {published data only}
Hussain 2007 {published data only}
-
- Hussain S, Gomez MM, Wludyka P, Chiu T, Rathore MH. Survival times and complications of catheters used for outpatient parenteral antibiotic therapy in children. Clinical Pediatrics 2007;46(3):247‐51. - PubMed
Inglis 2005 {published data only}
Kellam 1988 {published data only}
-
- Kellam B, Fraze DE, Kanarek KS. Central line dressing material and neonatal skin integrity. Nutrition in Clinical Practice 1988;3(2):65‐8. - PubMed
Larsen 2011 {published data only}
-
- Larsen LN, Malchau E, Kristensen B, Schroeder H. Hydrochloric acid treatment of tunneled central venous catheter infections in children with cancer. Journal of Pediatric Hematology/oncology 2011;33(2):e64‐8. - PubMed
Lee 2005 {published data only}
-
- Lee OK, Johnston L. A systematic review for effective management of central venous catheters and catheter sites in acute care paediatric patients. Worldviews on Evidence‐based Nursing / Sigma Theta Tau International, Honor Society of Nursing 2005;2(1):4‐13; discussion 14‐5. - PubMed
Leibovitz 1992 {published data only}
-
- Leibovitz E, Iuster‐Reicher A, Amitai M, Mogilner B. Systemic candidal infections associated with use of peripheral venous catheters in neonates: A 9‐year experience. Clinical Infectious Diseases 1992;14(2):485‐91. - PubMed
Li 2011 {published data only}
-
- Li S, Bizzarro MJ. Prevention of central line associated bloodstream infections in critical care units. Current Opinion in Pediatrics 2011;23(1):85‐90. - PubMed
Machado 2005 {published data only}
-
- Machado AF, Pedreira ML, Chaud MN. Prospective, randomized and controlled trial on the dwell time of peripheral intravenous catheters in children, according to three dressing regimens. Revista Latino‐Americana Enfermagem 2005;13(3):291‐8. - PubMed
Mahieu 2001 {published data only}
-
- Mahieu LM, Dooy JJ, Lenaerts AE, Ieven MM, Muynck AO. Catheter manipulations and the risk of catheter‐associated bloodstream infection in neonatal intensive care unit patients. Journal of Hospital Infection 2001;48(1):20‐6. - PubMed
Millar 2011 {published data only}
-
- Millar M, Zhou W, Skinner R, Pizer B, Hennessy E, Wilks M, et al. Accuracy of bacterial DNA testing for central venous catheter‐associated bloodstream infection in children with cancer. Health Technology Assessment 2011;15(7):1‐114. - PubMed
Quach 2014 {published data only}
-
- Quach C, Milstone AM, Perpete C, Bonenfant M, Moore DL, Perreault T. Chlorhexidine bathing in a tertiary care neonatal intensive care unit: Impact on central line‐associated bloodstream infections. Infection Control and Hospital Epidemiology 2014;35(2):158‐63. - PubMed
Ragavan 2010 {published data only}
-
- Ragavan M, Gazula S, Yadav DK, Agarwala S, Srinivas M, Bajpai M, et al. Peripherally inserted central venous lines versus central lines in surgical newborns ‐ A comparison. Indian Journal of Pediatrics 2010;77(2):171‐4. - PubMed
Sannoh 2010 {published data only}
-
- Sannoh S, Clones B, Munoz J, Montecalvo M, Parvez B. A multimodal approach to central venous catheter hub care can decrease catheter‐related bloodstream infection. American Journal of Infection Control 2010;38(6):424‐9. - PubMed
Seliem 2010 {published data only}
-
- Seliem W, Abdel‐Hady H, El‐Nady G. Amikacin‐heparin lock for prevention of catheter‐related bloodstream infection in neonates with extended umbilical venous catheters use: A randomized controlled trial. Journal of Neonatal‐Perinatal Medicine 2010;3(1):33‐41.
Shapey 2009 {published data only}
-
- Shapey IM, Foster MA, Whitehouse T, Jumaa P, Bion JF. Central venous catheter‐related bloodstream infections: improving post‐insertion catheter care. Journal of Hospital Infection 2009;71(2):117‐22. - PubMed
Spafford 1994 {published data only}
-
- Spafford PS, Sinkin RA, Cox C, Reubens L, Powell KR. Prevention of central venous catheter‐related coagulase‐negative staphylococcal sepsis in neonates. Journal of Pediatrics 1994;125(2):259‐63. - PubMed
Westergaard 2013 {published data only}
-
- Westergaard B, Classen V, Walther‐Larsen S. Peripherally inserted central catheters in infants and children ‐ Indications, techniques, complications and clinical recommendations. Acta Anaesthesiologica Scandinavica 2013;57(3):278‐87. - PubMed
Wielenga 2013 {published data only}
-
- Wielenga J, Hilberdink A, Hoogen A. A systematic literature review of dressing techniques for peripherally inserted central venous catheters (CVC) in neonatal intensive care units. Intensive Care Med 2013;39:S133.
Wilson 2007 {published data only}
-
- Wilson D, Verklan MT, Kennedy KA. Randomized trial of percutaneous central venous lines versus peripheral intravenous lines. Journal of Perinatology 2007;27(2):92‐6. - PubMed
Wirtschafter 2011 {published data only}
-
- Wirtschafter DD, Padilla G, Suh O, Wan K, Trupp D, Fayard EE. Antibiotic use for presumed neonatally acquired infections far exceeds that for central line‐associated blood stream infections: an exploratory critique. Journal of Perinatology 2011;31(8):514‐8. - PubMed
Additional references
Afsar 2009
-
- Afsar FS. Skin care for preterm and term neonates. Clinical and Experimental Dermatology 2009;34(8):855‐8. [PUBMED: 19575734] - PubMed
Arora 2010
Bashir 2012
-
- Bashir MH, Olson LK, Walters SA. Suppression of regrowth of normal skin flora under chlorhexidine gluconate dressings applied to chlorhexidine gluconate‐prepped skin. American Journal of Infection Control 2012;40(4):344‐8. - PubMed
Bizzarro 2010
-
- Bizzarro MJ, Sabo B, Noonan M, Bonfiglio MP, Northrup V, Diefenbach K. A quality improvement initiative to reduce central line‐associated bloodstream infections in a neonatal intensive care unit. Infection Control and Hospital Epidemiology 2010;31(3):241‐8. - PubMed
Brown 1989
-
- Brown E, Wenzel RP, Hendley JO. Exploration of the microbial anatomy of normal human skin by using plasmid profiles of coagulase‐negative staphylococci: search for the reservoir of resident skin flora. Journal of Infectious Diseases 1989;160(4):644‐50. - PubMed
CDC 2011
-
- O'Grady N, Alexander M, Burns LA, Dellinger P, Garland J, Heard SO, et al. Guidelines for the Prevention of Intravascular Catheter‐Related Infections, 2011. Centers for Disease Control and Prevention (CDC), Healthcare Infection Control Practices Advisory Committee (HICPAC) publications. http://www.cdc.gov/hicpac/pdf/guidelines/bsi‐guidelines‐2011.pdf (accessed 3 April 2014).
CDC 2014
-
- Central Line‐Associated Bloodstream Infection (CLABSI) Event. Centers for Disease Control and Prevention (CDC), Device‐associated module publication. http://www.cdc.gov/nhsn/PDFs/pscManual/4PSC_CLABScurrent.pdf (accessed 12 April 2014).
Chien 2002
-
- Chien LY, Macnab Y, Aziz K, Andrews W, McMillan DD, Lee SK. Variations in central venous catheter‐related infection risks among Canadian neonatal intensive care units. Pediatric Infectious Disease Journal 2002;21(6):505‐11. - PubMed
Cicalini 2004
Costello 2008
-
- Costello JM, Morrow DF, Graham DA, Potter‐Bynoe G, Sandora T, Laussen PC. Systematic intervention to reduce central line‐associated bloodstream infection rates in a pediatric cardiac intensive care unit. Pediatrics 2008;121(5):915‐23. - PubMed
Couto 2006
-
- Couto RC, Pedrosa TM, Tofani Cde P, Pedroso ER. Risk factors for nosocomial infection in a neonatal intensive care unit. Infection Control and Hospital Epidemiology 2006;27(6):571‐5. - PubMed
Fisher 2013
-
- Fisher D, Cochran KM, Provost LP, Patterson J, Bristol T, Metzguer K, et al. Reducing central line‐associated bloodstream infections in North Carolina NICUs. Pediatrics 2013;132(6):e1664‐71. [PUBMED: 24249819] - PubMed
Foster 2006
Gavin 2011
Ge 2012
Guyatt 2011a
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence‐‐study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] - PubMed
Guyatt 2011c
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] - PubMed
Guyatt 2011d
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] - PubMed
Guyatt 2011e
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence‐‐indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jaggi 2013
-
- Jaggi N, Rodrigues C, Rosenthal VD, Todi SK, Shah S, Saini N, et al. Impact of an international nosocomial infection control consortium multidimensional approach on central line‐associated bloodstream infection rates in adult intensive care units in eight cities in India. International Journal of Infectious Diseases 2013;17(12):e1218‐24. - PubMed
Jardine 2008
Kabra 2005
Karpanen 2009
Karpanen 2011
-
- Karpanen TJ, Casey AL, Conway BR, Lambert PA, Elliott TS. Antimicrobial activity of a chlorhexidine intravascular catheter site gel dressing. Journal of Antimicrobial Chemotherapy 2011;66(8):1777‐84. - PubMed
Lai 2012
Lai 2013
Leeming 1984
-
- Leeming JP, Holland KT, Cunliffe WJ. The microbial ecology of pilosebaceous units isolated from human skin. Journal of General Microbiology 1984;130(4):803‐7. - PubMed
Linares 2007
-
- Linares J. Diagnosis of catheter‐related bloodstream infection: conservative techniques. Clinical Infectious Diseases 2007; Vol. 44, issue 6:827‐9. [PUBMED: 17304455] - PubMed
Maki 1977
-
- Maki DG, Weise CE, Sarafin HW. A semiquantitative culture method for identifying intravenous‐catheter‐related infection. The New England Journal of Medicine 1977;296(23):1305‐9. - PubMed
McDonnell 1999
Olsen 2009
-
- Olsen AL, Reinholdt J, Jensen AM, Andersen LP, Jensen ET. Nosocomial infection in a Danish Neonatal Intensive Care Unit: a prospective study. Acta Paediatrica 2009;98(8):1294‐9. - PubMed
Pagani 2008
-
- Pagani JL, Eggimann P. Management of catheter‐related infection. Expert Review of Anti‐infective Therapy 2008;6(1):31‐7. - PubMed
Payne 2004
-
- Payne NR, Carpenter JH, Badger GJ, Horbar JD, Rogowski J. Marginal increase in cost and excess length of stay associated with nosocomial bloodstream infections in surviving very low birth weight infants. Pediatrics 2004;114(2):348‐55. [PUBMED: 15286215] - PubMed
Pratt 2007
Raad 2002
-
- Raad II, Hanna HA. Intravascular catheter‐related infections: new horizons and recent advances. Archives of Internal Medicine 2002;162(8):871‐8. [PUBMED: 11966337] - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rijnders 2002
-
- Rijnders BJ, Wijngaerden E, Peetermans WE. Catheter‐tip colonization as a surrogate end point in clinical studies on catheter‐related bloodstream infection: how strong is the evidence?. Clinical Infectious Diseases 2002;35(9):1053‐8. - PubMed
Rosenthal 2013
-
- Rosenthal VD, Duenas L, Sobreyra‐Oropeza M, Ammar K, Navoa‐Ng JA, Casares AC, et al. Findings of the International Nosocomial Infection Control Consortium (INICC), part III: effectiveness of a multidimensional infection control approach to reduce central line‐associated bloodstream infections in the neonatal intensive care units of 4 developing countries. Infection Control and Hospital Epidemiology 2013;34(3):229‐37. - PubMed
Schoot 2011
-
- Schoot RA, Dalen EC, Ommen CH, Wetering MD. Antibiotic and other lock treatments for tunnelled central venous catheter related infections in children with cancer. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2011, issue 2. [DOI: 10.1002/14651858.CD008975; CD008975] - DOI - PMC - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. Grade Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. Available from www.guidelinedevelopment.org/handbook Updated October 2013.
Sengupta 2010
Shah 2007
Taylor 2013
Ullman 2013
Vasudevan 2011
Washington 1998
-
- Washington K. The Bayley Scales of Infant Development‐II and children with developmental delays: a clinical perspective. Journal of Developmental and Behavioral Pediatrics 1998;19(5):346‐9. - PubMed
Webster 2011
Wong 2012
-
- Wong J, Dow K, Shah PS, Andrews W, Lee S. Percutaneously placed central venous catheter‐related sepsis in Canadian neonatal intensive care units. American Journal of Perinatology 2012;29(8):629‐34. - PubMed
Worthington 2005
-
- Worthington T, Elliott TS. Diagnosis of central venous catheter related infection in adult patients. Journal of Infection 2005;51(4):267‐80. - PubMed
Ygberg 2012
-
- Ygberg S, Nilsson A. The developing immune system ‐ from foetus to toddler. Acta Paediatrica 2012;101(2):120‐7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

