Robust Stimulation of W1282X-CFTR Channel Activity by a Combination of Allosteric Modulators
- PMID: 27007499
- PMCID: PMC4805204
- DOI: 10.1371/journal.pone.0152232
Robust Stimulation of W1282X-CFTR Channel Activity by a Combination of Allosteric Modulators
Abstract
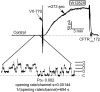
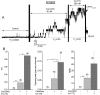
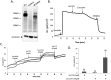
W1282X is a common nonsense mutation among cystic fibrosis patients that results in the production of a truncated Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) channel. Here we show that the channel activity of the W1282X-CFTR polypeptide is exceptionally low in excised membrane patches at normally saturating doses of ATP and PKA (single channel open probability (PO) < 0.01). However, W1282X-CFTR channels were stimulated by two CFTR modulators, the FDA-approved VX-770 and the dietary compound curcumin. Each of these compounds is an allosteric modulator of CFTR gating that promotes channel activity in the absence of the native ligand, ATP. Although W1282X-CFTR channels were stimulated by VX-770 in the absence of ATP their activities remained dependent on PKA phosphorylation. Thus, activated W1282X-CFTR channels should remain under physiologic control by cyclic nucleotide signaling pathways in vivo. VX-770 and curcumin exerted additive effects on W1282X-CFTR channel gating (opening/closing) in excised patches such that the Po of the truncated channel approached unity (> 0.9) when treated with both modulators. VX-770 and curcumin also additively stimulated W1282X-CFTR mediated currents in polarized FRT epithelial monolayers. In this setting, however, the stimulated W1282X-CFTR currents were smaller than those mediated by wild type CFTR (3-5%) due presumably to lower expression levels or cell surface targeting of the truncated protein. Combining allosteric modulators of different mechanistic classes is worth considering as a treatment option for W1282X CF patients perhaps when coupled with maneuvers to increase expression of the truncated protein.
Conflict of interest statement
Figures




References
-
- Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73(7):1251–4. . - PubMed
-
- Linde L, Boelz S, Nissim-Rafinia M, Oren YS, Wilschanski M, Yaacov Y, et al. Nonsense-mediated mRNA decay affects nonsense transcript levels and governs response of cystic fibrosis patients to gentamicin. The Journal of clinical investigation. 2007;117(3):683–92. 10.1172/JCI28523 - DOI - PMC - PubMed
-
- Berger AL, Ikuma M, Welsh MJ. Normal gating of CFTR requires ATP binding to both nucleotide-binding domains and hydrolysis at the second nucleotide-binding domain. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(2):455–60. 10.1073/pnas.0408575102 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

