Medroxyprogesterone acetate and levonorgestrel increase genital mucosal permeability and enhance susceptibility to genital herpes simplex virus type 2 infection
- PMID: 27007679
- PMCID: PMC5035233
- DOI: 10.1038/mi.2016.22
Medroxyprogesterone acetate and levonorgestrel increase genital mucosal permeability and enhance susceptibility to genital herpes simplex virus type 2 infection
Abstract
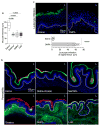
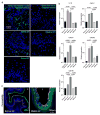
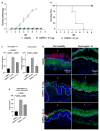
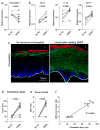
Depot-medroxyprogesterone acetate (DMPA) is a hormonal contraceptive especially popular in areas with high prevalence of HIV and other sexually transmitted infections (STI). Although observational studies identify DMPA as an important STI risk factor, mechanisms underlying this connection are undefined. Levonorgestrel (LNG) is another progestin used for hormonal contraception, but its effect on STI susceptibility is much less explored. Using a mouse model of genital herpes simplex virus type 2 (HSV-2) infection, we herein found that DMPA and LNG similarly reduced genital expression of the desmosomal cadherin desmoglein-1α (DSG1α), enhanced access of inflammatory cells to genital tissue by increasing mucosal epithelial permeability, and increased susceptibility to viral infection. Additional studies with uninfected mice revealed that DMPA-mediated increases in mucosal permeability promoted tissue inflammation by facilitating endogenous vaginal microbiota invasion. Conversely, concomitant treatment of mice with DMPA and intravaginal estrogen restored mucosal barrier function and prevented HSV-2 infection. Evaluating ectocervical biopsy tissue from women before and 1 month after initiating DMPA remarkably revealed that inflammation and barrier protection were altered by treatment identically to changes seen in progestin-treated mice. Together, our work reveals DMPA and LNG diminish the genital mucosal barrier; a first-line defense against all STI, but may offer foundation for new contraceptive strategies less compromising of barrier protection.
Conflict of interest statement
Authors have no conflict of interests to declare.
Figures


References
-
- Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science. 2010;308:1582–1583. - PubMed
-
- Morrison CS, et al. Hormonal contraceptive use, cervical ectopy, and the acquisition of cervical infections. Sex Transm Dis. 2004;31:561–567. - PubMed
-
- Baeten JM, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS. 2007;21:1771–1777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

