Primary cilia are not calcium-responsive mechanosensors
- PMID: 27007841
- PMCID: PMC4851444
- DOI: 10.1038/nature17426
Primary cilia are not calcium-responsive mechanosensors
Abstract
Primary cilia are solitary, generally non-motile, hair-like protrusions that extend from the surface of cells between cell divisions. Their antenna-like structure leads naturally to the assumption that they sense the surrounding environment, the most common hypothesis being sensation of mechanical force through calcium-permeable ion channels within the cilium. This Ca(2+)-responsive mechanosensor hypothesis for primary cilia has been invoked to explain a large range of biological responses, from control of left-right axis determination in embryonic development to adult progression of polycystic kidney disease and some cancers. Here we report the complete lack of mechanically induced calcium increases in primary cilia, in tissues upon which this hypothesis has been based. We developed a transgenic mouse, Arl13b-mCherry-GECO1.2, expressing a ratiometric genetically encoded calcium indicator in all primary cilia. We then measured responses to flow in primary cilia of cultured kidney epithelial cells, kidney thick ascending tubules, crown cells of the embryonic node, kinocilia of inner ear hair cells, and several cell lines. Cilia-specific Ca(2+) influxes were not observed in physiological or even highly supraphysiological levels of fluid flow. We conclude that mechanosensation, if it originates in primary cilia, is not via calcium signalling.
Figures
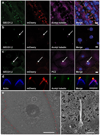
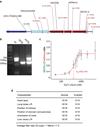

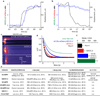
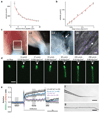
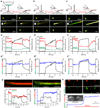
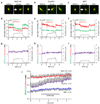

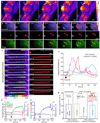
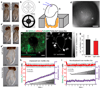



Comment in
-
Cell biology: Calcium contradictions in cilia.Nature. 2016 Mar 31;531(7596):582-3. doi: 10.1038/nature17313. Epub 2016 Mar 23. Nature. 2016. PMID: 27007852 No abstract available.
-
Polycystic kidney disease: Cilia and mechanosensation revisited.Nat Rev Nephrol. 2016 Jun;12(6):318-9. doi: 10.1038/nrneph.2016.61. Epub 2016 May 3. Nat Rev Nephrol. 2016. PMID: 27140854 No abstract available.
References
-
- Hamada H. Role of physical forces in embryonic development. Semin Cell Dev Biol. 2015;47–48:88–91. - PubMed
-
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. - PubMed
-
- Corey DP, Hudspeth AJ. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979;281:675–677. - PubMed
References for Methods and SI
-
- Takao D, Nemoto T, Abe T, Kiyonari H, Kajiura-Kobayashi H, Shiratori H, Nonaka S. Asymmetric distribution of dynamic calcium signals in the node of mouse embryo during left-right axis formation. Dev Biol. 2013;376:23–30. - PubMed
-
- Downs KM, Davies T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development. 1993;118:1255–1266. - PubMed
-
- Yoshiba S, Hamada H. Roles of cilia, fluid flow, and Ca2+ signaling in breaking of left-right symmetry. Trends Genet. 2014;30:10–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

