A Novel Sample Processing Method for Rapid Detection of Tuberculosis in the Stool of Pediatric Patients Using the Xpert MTB/RIF Assay
- PMID: 27007974
- PMCID: PMC4805262
- DOI: 10.1371/journal.pone.0151980
A Novel Sample Processing Method for Rapid Detection of Tuberculosis in the Stool of Pediatric Patients Using the Xpert MTB/RIF Assay
Abstract
Background: Tuberculosis (TB) is difficult to diagnose in children using molecular tests, because children have difficulty providing respiratory samples. Stool could replace sputum for diagnostic TB testing if adequate sample processing techniques were available.
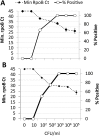
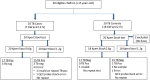
Methods: We developed a rapid method to process large volumes of stool for downstream testing by the Xpert MTB/RIF (Xpert) TB-detection assay. The method was tested and optimized on stool samples spiked with known numbers of M. tuberculosis colony forming units (CFU), and stools from M. tuberculosis-infected cynomolgus macaques (Macaca fascicularis). Performance was scored on number of positive Xpert tests, the cycle thresholds (Cts) of the Xpert sample-processing control (SPC), and the Cts of the M. tuberculosis-specific rpoB probes. The method was then validated on 20 confirmed TB cases and 20 controls in Durban, South Africa.
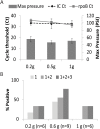
Results: The assay's analytical limit of detection was 1,000 CFU/g of stool. As much as one gram of spiked stool could be tested without showing increased PCR inhibition. In analytical spiking experiments using human stool, 1g samples provided the best sensitivity compared to smaller amounts of sample. However, in Macaques with TB, 0.6g stool samples performed better than either 0.2g or 1.2g samples. Testing the stool of pediatric TB suspects and controls suggested an assay sensitivity of 85% (95% CI 0.6-0.9) and 84% (95% CI 0.6-0.96) for 0.6g and 1.2g stool samples, respectively, and a specificity of 100% (95% CI 0.77-1) and 94% (95% CI 0.7-0.99), respectively.
Conclusion: This novel approach may permit simple and rapid detection of TB using pediatric stool samples.
Conflict of interest statement
Figures

References
-
- WHO. Roadmap for childhood tuberculosis: towards zero deaths. Geneva, Switzerland.: WHO press; 2013.
-
- WHO. Global Tuberculosis Report. 2014.
-
- Cuevas LE, Browning R, Bossuyt P, Casenghi M, Cotton MF, Cruz AT, et al. Evaluation of tuberculosis diagnostics in children: 2. Methodological issues for conducting and reporting research evaluations of tuberculosis diagnostics for intrathoracic tuberculosis in children. Consensus from an expert panel. J Infect Dis. 2012;205 Suppl 2:S209–15. 10.1093/infdis/jir879 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

