Anti-inflammatory Effect of Mesenchymal Stromal Cell Transplantation and Quercetin Treatment in a Rat Model of Experimental Cerebral Ischemia
- PMID: 27008429
- PMCID: PMC11482404
- DOI: 10.1007/s10571-015-0291-6
Anti-inflammatory Effect of Mesenchymal Stromal Cell Transplantation and Quercetin Treatment in a Rat Model of Experimental Cerebral Ischemia
Abstract
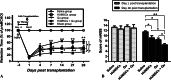
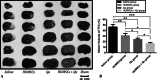
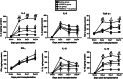
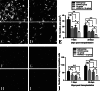
Here, we have investigated the synergistic effect of quercetin administration and transplantation of human umbilical cord mesenchymal stromal cells (HUMSCs) following middle cerebral artery occlusion in rat. Combining quercetin treatment with delayed transplantation of HUMSCs after local cerebral ischemia significantly (i) improved neurological functional recovery; (ii) reduced proinflammatory cytokines (interleukin(IL)-1β and IL-6), increased anti-inflammatory cytokines (IL-4, IL-10, and transforming growth factor-β1), and reduced ED-1 positive areas; (iii) inhibited cell apoptosis (caspase-3 expression); and (iv) improved the survival rate of HUMSCs in the injury site. Altogether, our results demonstrate that combined HUMSC transplantation and quercetin treatment is a potential strategy for reducing secondary damage and promoting functional recovery following cerebral ischemia.
Keywords: Apoptosis; Inflammation; Mesenchymal stromal cells; Quercetin; Stroke.
Figures



Similar articles
-
Combinatory effect of mesenchymal stromal cells transplantation and quercetin after spinal cord injury in rat.Eur Rev Med Pharmacol Sci. 2018 May;22(9):2876-2887. doi: 10.26355/eurrev_201805_14990. Eur Rev Med Pharmacol Sci. 2018. PMID: 29771441
-
Interleukin-1 receptor antagonist-mediated neuroprotection by umbilical cord-derived mesenchymal stromal cells following transplantation into a rodent stroke model.Exp Mol Med. 2018 Apr 13;50(4):1-12. doi: 10.1038/s12276-018-0041-1. Exp Mol Med. 2018. PMID: 29650950 Free PMC article.
-
Reversal of bleomycin-induced rat pulmonary fibrosis by a xenograft of human umbilical mesenchymal stem cells from Wharton's jelly.Theranostics. 2019 Sep 17;9(22):6646-6664. doi: 10.7150/thno.33741. eCollection 2019. Theranostics. 2019. PMID: 31588241 Free PMC article.
-
The role of small extracellular vesicles in cerebral and myocardial ischemia-Molecular signals, treatment targets, and future clinical translation.Stem Cells. 2021 Apr;39(4):403-413. doi: 10.1002/stem.3329. Epub 2021 Jan 12. Stem Cells. 2021. PMID: 33432732 Review.
-
Neuroprotection by mesenchymal stem cell (MSC) administration is enhanced by local cooling infusion (LCI) in ischemia.Brain Res. 2019 Dec 1;1724:146406. doi: 10.1016/j.brainres.2019.146406. Epub 2019 Aug 24. Brain Res. 2019. PMID: 31454517 Review.
Cited by
-
Potential Mechanisms and Perspectives in Ischemic Stroke Treatment Using Stem Cell Therapies.Front Cell Dev Biol. 2021 Apr 1;9:646927. doi: 10.3389/fcell.2021.646927. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33869200 Free PMC article. Review.
-
Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms.Prog Neurobiol. 2017 Nov;158:94-131. doi: 10.1016/j.pneurobio.2017.07.004. Epub 2017 Jul 23. Prog Neurobiol. 2017. PMID: 28743464 Free PMC article. Review.
-
Analysis of the Anti-Inflammatory and Analgesic Mechanism of Shiyifang Vinum Based on Network Pharmacology.Evid Based Complement Alternat Med. 2021 Jan 13;2021:8871276. doi: 10.1155/2021/8871276. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 33519947 Free PMC article.
-
Neuroprotective Effects of Bioactive Compounds and MAPK Pathway Modulation in "Ischemia"-Stressed PC12 Pheochromocytoma Cells.Brain Sci. 2018 Feb 8;8(2):32. doi: 10.3390/brainsci8020032. Brain Sci. 2018. PMID: 29419806 Free PMC article. Review.
-
Prediction of the Mechanism of Shaoyao Gancao Decoction in the Treatment of Alopecia Areata by Network Pharmacology and Its Preliminary Verification Study.Evid Based Complement Alternat Med. 2022 Apr 7;2022:5764107. doi: 10.1155/2022/5764107. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35432570 Free PMC article.
References
-
- Anderson AJ (2002) Mechanisms and pathways of inflammatory responses in CNS trauma: spinal cord injury. J Spinal Cord Med 25(2):70–79 discussion 80 - PubMed
-
- Bednar MM, Gross CE (1999) Antiplatelet therapy in acute cerebral ischemia. Stroke 30(4):887–893 - PubMed
-
- Bosoi CR, Rose CF (2013) Oxidative stress: a systemic factor implicated in the pathogenesis of hepatic encephalopathy. Metab Brain Dis 28(2):175–178. doi:10.1007/s11011-012-9351-5 - PubMed
-
- Bosoi CR, Yang X, Huynh J, Parent-Robitaille C, Jiang W, Tremblay M, Rose CF (2012) Systemic oxidative stress is implicated in the pathogenesis of brain edema in rats with chronic liver failure. Free Radic Biol Med 52(7):1228–1235. doi:10.1016/j.freeradbiomed.2012.01.006 - PubMed
-
- Chelluboina B, Klopfenstein JD, Pinson DM, Wang DZ, Veeravalli KK (2014) Stem cell treatment after cerebral ischemia regulates the gene expression of apoptotic molecules. Neurochem Res 39(8):1511–1521. doi:10.1007/s11064-014-1341-z - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

