Muscle Tissue Engineering Using Gingival Mesenchymal Stem Cells Encapsulated in Alginate Hydrogels Containing Multiple Growth Factors
- PMID: 27009085
- PMCID: PMC4880526
- DOI: 10.1007/s10439-016-1594-6
Muscle Tissue Engineering Using Gingival Mesenchymal Stem Cells Encapsulated in Alginate Hydrogels Containing Multiple Growth Factors
Abstract
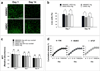
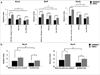
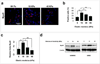
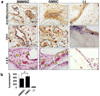
Repair and regeneration of muscle tissue following traumatic injuries or muscle diseases often presents a challenging clinical situation. If a significant amount of tissue is lost the native regenerative potential of skeletal muscle will not be able to grow to fill the defect site completely. Dental-derived mesenchymal stem cells (MSCs) in combination with appropriate scaffold material, present an advantageous alternative therapeutic option for muscle tissue engineering in comparison to current treatment modalities available. To date, there has been no report on application of gingival mesenchymal stem cells (GMSCs) in three-dimensional scaffolds for muscle tissue engineering. The objectives of the current study were to develop an injectable 3D RGD-coupled alginate scaffold with multiple growth factor delivery capacity for encapsulating GMSCs, and to evaluate the capacity of encapsulated GMSCs to differentiate into myogenic tissue in vitro and in vivo where encapsulated GMSCs were transplanted subcutaneously into immunocompromised mice. The results demonstrate that after 4 weeks of differentiation in vitro, GMSCs as well as the positive control human bone marrow mesenchymal stem cells (hBMMSCs) exhibited muscle cell-like morphology with high levels of mRNA expression for gene markers related to muscle regeneration (MyoD, Myf5, and MyoG) via qPCR measurement. Our quantitative PCR analyzes revealed that the stiffness of the RGD-coupled alginate regulates the myogenic differentiation of encapsulated GMSCs. Histological and immunohistochemical/fluorescence staining for protein markers specific for myogenic tissue confirmed muscle regeneration in subcutaneous transplantation in our in vivo animal model. GMSCs showed significantly greater capacity for myogenic regeneration in comparison to hBMMSCs (p < 0.05). Altogether, our findings confirmed that GMSCs encapsulated in RGD-modified alginate hydrogel with multiple growth factor delivery capacity is a promising candidate for muscle tissue engineering.
Keywords: Dental mesenchymal stem cells; Muscle regeneration; RGD-coupled alginate hydrogel; Tissue engineering.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures



References
-
- Adnot S, Desmier M, Ferry N, Hanoune J, Sevenet T. Forskolin (a powerful inhibitor of human platelet aggregation) Biochem. Pharmacol. 1982;31:4071–4074. - PubMed
-
- Abou-Khalil R, Yang F, Lieu S, Julien A, Perry J, Pereira C, Relaix F, Miclau T, Marcucio R, Colnot C. Role of muscle stem cells during skeletal regeneration. Stem Cells. 2015;33:1501–1511. - PubMed
-
- Beier JP, Bitto FF, Lange C, Klumpp D, Arkudas A, Bleiziffer O O. Myogenic differentiation of mesenchymal stem cells co-cultured with primary myoblasts. Cell. Biol. Int. 2011;35:397–406. - PubMed
-
- Boontheekula T, Kongc HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26:2455–2465. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

