Mechanisms of HIV Neuropathogenesis: Role of Cellular Communication Systems
- PMID: 27009098
- PMCID: PMC5052113
- DOI: 10.2174/1570162x14666160324124558
Mechanisms of HIV Neuropathogenesis: Role of Cellular Communication Systems
Abstract
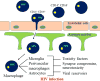
Background: One of the major complications of Human Immunodeficiency Virus (HIV) infection is the development of HIV-Associated Neurocognitive Disorders (HANDs) in approximately 50-60% of HIV infected individuals. Despite undetectable viral loads in the periphery owing to anti-retroviral therapy, neuroinflammation and neurocognitive impairment are still prevalent in HIV infected individuals. Several studies indicate that the central nervous system (CNS) abnormalities observed in HIV infected individuals are not a direct effect of viral replication in the CNS, rather these neurological abnormalities are associated with amplification of HIV specific signals by unknown mechanisms. We propose that some of these mechanisms of damage amplification are mediated by gap junction channels, pannexin and connexin hemichannels, tunneling nanotubes and microvesicles/exosomes.
Objective: Our laboratory and others have demonstrated that HIV infection targets cell to cell communication by altering all these communication systems resulting in enhanced bystander apoptosis of uninfected cells, inflammation and viral infection. Here we discuss the role of these communication systems in HIV neuropathogenesis.
Conclusion: In the current manuscript, we have described the mechanisms by which HIV "hijacks" these host cellular communication systems, leading to exacerbation of HIV neuropathogenesis, and to simultaneously promote the survival of HIV infected cells, resulting in the establishment of viral reservoirs.
Conflict of interest statement
The authors confirm that this article content has no conflict of interest.
Figures


Similar articles
-
The impact of substance abuse on HIV-mediated neuropathogenesis in the current ART era.Brain Res. 2019 Dec 1;1724:146426. doi: 10.1016/j.brainres.2019.146426. Epub 2019 Aug 29. Brain Res. 2019. PMID: 31473221 Free PMC article. Review.
-
Connexin43 Containing Gap Junction Channels Facilitate HIV Bystander Toxicity: Implications in NeuroHIV.Front Mol Neurosci. 2017 Dec 5;10:404. doi: 10.3389/fnmol.2017.00404. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29259541 Free PMC article.
-
HIV-1 Induced CNS Dysfunction: Current Overview and Research Priorities.Curr HIV Res. 2016;14(5):389-399. doi: 10.2174/1570162x14666160324124940. Curr HIV Res. 2016. PMID: 27009096 Review.
-
Role of Connexin and Pannexin containing channels in HIV infection and NeuroAIDS.Neurosci Lett. 2019 Mar 16;695:86-90. doi: 10.1016/j.neulet.2017.09.005. Epub 2017 Sep 5. Neurosci Lett. 2019. PMID: 28886986 Free PMC article. Review.
-
Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus.Virus Res. 2005 Aug;111(2):194-213. doi: 10.1016/j.virusres.2005.04.009. Virus Res. 2005. PMID: 15885841 Review.
Cited by
-
Exosomes derived from HIV-1-infected cells promote growth and progression of cancer via HIV TAR RNA.Nat Commun. 2018 Nov 2;9(1):4585. doi: 10.1038/s41467-018-07006-2. Nat Commun. 2018. PMID: 30389917 Free PMC article.
-
The Glutamate System as a Crucial Regulator of CNS Toxicity and Survival of HIV Reservoirs.Front Cell Infect Microbiol. 2020 Jun 24;10:261. doi: 10.3389/fcimb.2020.00261. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32670889 Free PMC article. Review.
-
HIV infection and latency induce a unique metabolic signature in human macrophages.Sci Rep. 2019 Mar 8;9(1):3941. doi: 10.1038/s41598-019-39898-5. Sci Rep. 2019. PMID: 30850623 Free PMC article.
-
Darunavir Nanoformulation Suppresses HIV Pathogenesis in Macrophages and Improves Drug Delivery to the Brain in Mice.Pharmaceutics. 2024 Apr 19;16(4):555. doi: 10.3390/pharmaceutics16040555. Pharmaceutics. 2024. PMID: 38675216 Free PMC article.
-
JC Polyomavirus Attachment and Entry: Potential Sites for PML Therapeutics.Curr Clin Microbiol Rep. 2017 Sep;4(3):132-141. doi: 10.1007/s40588-017-0069-3. Epub 2017 Aug 1. Curr Clin Microbiol Rep. 2017. PMID: 28989857 Free PMC article.
References
-
- Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. Journal of neurovirology. 2004;10(6):350–7. - PubMed
-
- McArthur JC, McDermott MP, McClernon D, St Hillaire C, Conant K, Marder K, et al. Attenuated central nervous system infection in advanced HIV/AIDS with combination antiretroviral therapy. Archives of neurology. 2004;61(11):1687–96. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
