Engineering a more sustainable world through catalysis and green chemistry
- PMID: 27009181
- PMCID: PMC4843682
- DOI: 10.1098/rsif.2016.0087
Engineering a more sustainable world through catalysis and green chemistry
Abstract
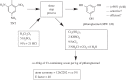
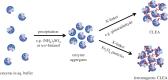
The grand challenge facing the chemical and allied industries in the twenty-first century is the transition to greener, more sustainable manufacturing processes that efficiently use raw materials, eliminate waste and avoid the use of toxic and hazardous materials. It requires a paradigm shift from traditional concepts of process efficiency, focusing on chemical yield, to one that assigns economic value to replacing fossil resources with renewable raw materials, eliminating waste and avoiding the use of toxic and/or hazardous substances. The need for a greening of chemicals manufacture is readily apparent from a consideration of the amounts of waste generated per kilogram of product (the E factors) in various segments of the chemical industry. A primary source of this waste is the use of antiquated 'stoichiometric' technologies and a major challenge is to develop green, catalytic alternatives. Another grand challenge for the twenty-first century, driven by the pressing need for climate change mitigation, is the transition from an unsustainable economy based on fossil resources--oil, coal and natural gas--to a sustainable one based on renewable biomass. In this context, the valorization of waste biomass, which is currently incinerated or goes to landfill, is particularly attractive. The bio-based economy involves cross-disciplinary research at the interface of biotechnology and chemical engineering, focusing on the development of green, chemo- and biocatalytic technologies for waste biomass conversion to biofuels, chemicals and bio-based materials. Biocatalysis has many benefits to offer in this respect. The catalyst is derived from renewable biomass and is biodegradable. Processes are performed under mild conditions and generally produce less waste and are more energy efficient than conventional ones. Thanks to modern advances in biotechnology 'tailor-made' enzymes can be economically produced on a large scale. However, for economic viability it is generally necessary to recover and re-use the enzyme and this can be achieved by immobilization, e.g. as solid cross-linked enzyme aggregates (CLEAs), enabling separation by filtration or centrifugation. A recent advance is the use of 'smart', magnetic CLEAs, which can be separated magnetically from reaction mixtures containing suspensions of solids; truly an example of cross-disciplinary research at the interface of physical and life sciences, which is particularly relevant to biomass conversion processes.
Keywords: biocatalysis; biomass conversion; catalysis; green chemistry; immobilized enzymes; sustainability.
© 2016 The Author(s).
Figures





Similar articles
-
Biocatalysis and biomass conversion: enabling a circular economy.Philos Trans A Math Phys Eng Sci. 2020 Jul 24;378(2176):20190274. doi: 10.1098/rsta.2019.0274. Epub 2020 Jul 6. Philos Trans A Math Phys Eng Sci. 2020. PMID: 32623984
-
Fundamentals of green chemistry: efficiency in reaction design.Chem Soc Rev. 2012 Feb 21;41(4):1437-51. doi: 10.1039/c1cs15219j. Epub 2011 Oct 28. Chem Soc Rev. 2012. PMID: 22033698 Review.
-
Generation, capture, and utilization of industrial carbon dioxide.ChemSusChem. 2010 Mar 22;3(3):306-22. doi: 10.1002/cssc.200900169. ChemSusChem. 2010. PMID: 20049768 Review.
-
Crops: a green approach toward self-assembled soft materials.Acc Chem Res. 2008 Jun;41(6):769-82. doi: 10.1021/ar7002682. Acc Chem Res. 2008. PMID: 18507403
-
Recirculation: A New Concept to Drive Innovation in Sustainable Product Design for Bio-Based Products.Molecules. 2016 Dec 29;22(1):48. doi: 10.3390/molecules22010048. Molecules. 2016. PMID: 28036077 Free PMC article.
Cited by
-
Surface coverage control for dramatic enhancement of thermal CO oxidation by precise potential tuning of metal supported catalysts.Chem Sci. 2022 Aug 11;13(33):9774-9783. doi: 10.1039/d2sc03145k. eCollection 2022 Aug 24. Chem Sci. 2022. PMID: 36091892 Free PMC article.
-
Immobilization of Glycoside Hydrolase Families GH1, GH13, and GH70: State of the Art and Perspectives.Molecules. 2016 Aug 17;21(8):1074. doi: 10.3390/molecules21081074. Molecules. 2016. PMID: 27548117 Free PMC article. Review.
-
Forizymes - functionalised artificial forisomes as a platform for the production and immobilisation of single enzymes and multi-enzyme complexes.Sci Rep. 2016 Aug 9;6:30839. doi: 10.1038/srep30839. Sci Rep. 2016. PMID: 27502156 Free PMC article.
-
A Visible-Light Promoted Amine Oxidation Catalyzed by a Cp*Ir Complex.ChemCatChem. 2020 Sep 17;12(18):4512-4516. doi: 10.1002/cctc.202000488. Epub 2020 Jul 22. ChemCatChem. 2020. PMID: 33777249 Free PMC article.
-
A Sustainable Green Enzymatic Method for Amide Bond Formation.Molecules. 2023 Jul 28;28(15):5706. doi: 10.3390/molecules28155706. Molecules. 2023. PMID: 37570676 Free PMC article.
References
-
- Sheldon RA, Arends IWCE, Hanefeld U. 2007. Green chemistry and catalysis. Weinheim, Germany: Wiley-VCH.
-
- Anastas PT, Warner JC. 1998. Green chemistry: theory and practice. Oxford, UK: Oxford University Press.
-
- Sheldon RA. 2007. The E factor: fifteen years on. Green Chem. 9, 1273–1283. (10.1039/b713736m) - DOI
-
- Sheldon RA. 1992. Organic synthesis: past, present and future. Chem. Ind. (Lond.) 23, 903–906.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

