Autocrine VEGFR1 and VEGFR2 signaling promotes survival in human glioblastoma models in vitro and in vivo
- PMID: 27009237
- PMCID: PMC4998998
- DOI: 10.1093/neuonc/now043
Autocrine VEGFR1 and VEGFR2 signaling promotes survival in human glioblastoma models in vitro and in vivo
Abstract
Background: Although the vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) system has become a prime target for antiangiogenic treatment, its biological role in glioblastoma beyond angiogenesis has remained controversial.
Methods: Using neutralizing antibodies to VEGF or placental growth factor (PlGF) or the tyrosine kinase inhibitor, cediranib, or lentiviral gene silencing, we delineated autocrine signaling in glioma cell lines. The in vivo effects of VEGFR1 and VEGFR2 depletion were evaluated in orthotopic glioma xenograft models.
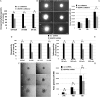
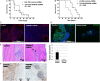
Results: VEGFR1 and VEGFR2 modulated glioma cell clonogenicity, viability, and invasiveness in vitro in an autocrine, cell-line-specific manner. VEGFR1 silencing promoted mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling, whereas VEGFR2 silencing resulted in cell-type dependent activation of the protein kinase B (PKB)/AKT and MAPK/ERK pathways. These responses may represent specific escape mechanisms from VEGFR inhibition. The survival of orthotopic glioma-bearing mice was prolonged upon VEGFR1 silencing in the LNT-229, LN-308, and U87MG models and upon VEGFR2 silencing in LN-308 and U87MG. Disruption of VEGFR1 and VEGFR2 signaling was associated with decreased tumor size, increased tumor necrosis, or loss of matrix metalloproteinase 9 (MMP9) immunoreactivity. Neutralizing VEGF and PlGF by specific antibodies was superior to either antibody treatment alone in the VEGFR1-dependent LNT-229 model.
Conclusions: Differential dependence on autocrine signaling through VEGFR1 and VEGFR2 suggests a need for biomarker-stratified VEGF(R)-based therapeutic approaches to glioblastoma.
Keywords: PlGF; VEGF; angiogenesis; glioblastoma; signaling.
© The Author(s) 2016. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Figures




References
-
- Plate KH, Breier G, Weich HA et al. . Vascular endothelial growth-factor and glioma angiogenesis - coordinate induction of VEGF receptors, distribution of VEGF protein and possible in-vivo regulatory mechanisms. Int J Cancer. 1994;59 (4):520–529. - PubMed
-
- Nomura M, Yamagishi S, Harada S et al. . Placenta growth factor (PlGF) mRNA expression in brain tumors. J Neurooncol. 1998;40 (2):123–130. - PubMed
-
- Carmeliet P, Moons L, Luttun A et al. . Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7 (5):575–583. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

