Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis
- PMID: 27009267
- PMCID: PMC5074090
- DOI: 10.1126/scitranslmed.aad7151
Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis
Abstract
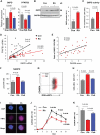
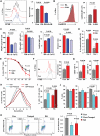
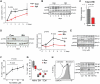
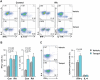
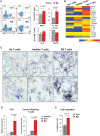
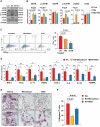
In patients with rheumatoid arthritis (RA), CD4(+)T cells hyperproliferate during clonal expansion, differentiating into cytokine-producing effector cells that contribute to disease pathology. However, the metabolic underpinnings of this hyperproliferation remain unclear. In contrast to healthy T cells, naïve RA T cells had a defect in glycolytic flux due to the up-regulation of glucose-6-phosphate dehydrogenase (G6PD). Excess G6PD shunted glucose into the pentose phosphate pathway, resulting in NADPH (reduced form of nicotinamide adenine dinucleotide phosphate) accumulation and reactive oxygen species (ROS) consumption. With surplus reductive equivalents, RA T cells insufficiently activated the redox-sensitive kinase ataxia telangiectasia mutated (ATM), bypassed the G2/M cell cycle checkpoint, and hyperproliferated. Moreover, insufficient ATM activation biased T cell differentiation toward the T helper 1 (TH1) and TH17 lineages, imposing a hyperinflammatory phenotype. We have identified several interventions that replenish intracellular ROS, which corrected the abnormal proliferative behavior of RA T cells and successfully suppressed synovial inflammation. Thus, rebalancing glucose utilization and restoring oxidant signaling may provide a therapeutic strategy to prevent autoimmunity in RA.
Copyright © 2016, American Association for the Advancement of Science.
Figures

Comment in
-
Metabolic control of arthritis: Switch pathways to treat.Sci Transl Med. 2016 Mar 23;8(331):331fs8. doi: 10.1126/scitranslmed.aaf4953. Sci Transl Med. 2016. PMID: 27009266
-
Rheumatoid arthritis: Metabolic interventions repair CD4(+) T cells in RA.Nat Rev Rheumatol. 2016 May;12(5):254. doi: 10.1038/nrrheum.2016.54. Epub 2016 Apr 15. Nat Rev Rheumatol. 2016. PMID: 27080694 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- AI044142/AI/NIAID NIH HHS/United States
- I01 BX001669/BX/BLRD VA/United States
- R01 AR042527/AR/NIAMS NIH HHS/United States
- R01 AI044142/AI/NIAID NIH HHS/United States
- P01 HL058000/HL/NHLBI NIH HHS/United States
- R01 AG045779/AG/NIA NIH HHS/United States
- R01 AI108906/AI/NIAID NIH HHS/United States
- AI108906/AI/NIAID NIH HHS/United States
- R56 AI044142/AI/NIAID NIH HHS/United States
- R01 AI108891/AI/NIAID NIH HHS/United States
- AI108891/AI/NIAID NIH HHS/United States
- AR042527/AR/NIAMS NIH HHS/United States
- HL058000/HL/NHLBI NIH HHS/United States
- AG045779/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

