Comparison between direct and reverse electroporation of cells in situ: a simulation study
- PMID: 27009275
- PMCID: PMC4814886
- DOI: 10.14814/phy2.12673
Comparison between direct and reverse electroporation of cells in situ: a simulation study
Abstract
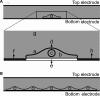
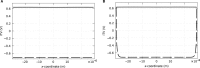
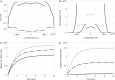
The discovery of the human genome has unveiled new fields of genomics, transcriptomics, and proteomics, which has produced paradigm shifts on how to study disease mechanisms, wherein a current central focus is the understanding of how gene signatures and gene networks interact within cells. These gene function studies require manipulating genes either through activation or inhibition, which can be achieved by temporarily permeabilizing the cell membrane through transfection to delivercDNAorRNAi. An efficient transfection technique is electroporation, which applies an optimized electric pulse to permeabilize the cells of interest. When the molecules are applied on top of seeded cells, it is called "direct" transfection and when the nucleic acids are printed on the substrate and the cells are seeded on top of them, it is termed "reverse" transfection. Direct transfection has been successfully applied in previous studies, whereas reverse transfection has recently gained more attention in the context of high-throughput experiments. Despite the emerging importance, studies comparing the efficiency of the two methods are lacking. In this study, a model for electroporation of cells in situ is developed to address this deficiency. The results indicate that reverse transfection is less efficient than direct transfection. However, the model also predicts that by increasing the concentration of deliverable molecules by a factor of 2 or increasing the applied voltage by 20%, reverse transfection can be approximately as efficient as direct transfection.
Keywords: Electroporation; high‐throughput techniques; transfection efficiency.
© 2016 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures




Similar articles
-
High efficiency, site-specific transfection of adherent cells with siRNA using microelectrode arrays (MEA).J Vis Exp. 2012 Sep 13;(67):e4415. doi: 10.3791/4415. J Vis Exp. 2012. PMID: 23007885 Free PMC article.
-
Efficient transfection of endothelial cells by a double-pulse electroporation method.DNA Cell Biol. 2009 Nov;28(11):561-6. doi: 10.1089/dna.2009.0915. DNA Cell Biol. 2009. PMID: 19630533
-
Substrate-mediated, high-efficiency siRNA electroporation.Methods Mol Biol. 2014;1121:139-46. doi: 10.1007/978-1-4614-9632-8_12. Methods Mol Biol. 2014. PMID: 24510819
-
Electroporation Knows No Boundaries: The Use of Electrostimulation for siRNA Delivery in Cells and Tissues.J Biomol Screen. 2015 Sep;20(8):932-42. doi: 10.1177/1087057115579638. Epub 2015 Apr 7. J Biomol Screen. 2015. PMID: 25851034 Free PMC article. Review.
-
Single cell optical transfection.J R Soc Interface. 2010 Jun 6;7(47):863-71. doi: 10.1098/rsif.2009.0463. Epub 2010 Jan 11. J R Soc Interface. 2010. PMID: 20064901 Free PMC article. Review.
Cited by
-
Investigation of ac-magnetic field stimulated nanoelectroporation of magneto-electric nano-drug-carrier inside CNS cells.Sci Rep. 2017 Apr 4;7:45663. doi: 10.1038/srep45663. Sci Rep. 2017. PMID: 28374799 Free PMC article.
-
Translocation and fate of nanospheres in pheochromocytoma cells following exposure to synchrotron-sourced terahertz radiation.J Synchrotron Radiat. 2023 Jul 1;30(Pt 4):780-787. doi: 10.1107/S1600577523004228. Epub 2023 Jun 20. J Synchrotron Radiat. 2023. PMID: 37338043 Free PMC article.
-
Translocation of silica nanospheres through giant unilamellar vesicles (GUVs) induced by a high frequency electromagnetic field.RSC Adv. 2021 Sep 23;11(50):31408-31420. doi: 10.1039/d1ra05459g. eCollection 2021 Sep 21. RSC Adv. 2021. PMID: 35496859 Free PMC article.
-
Temperature-Dependent Cytokine Neutralization Induced by Magnetoelectric Nanoparticles: An In Silico Study.Int J Mol Sci. 2024 Dec 19;25(24):13591. doi: 10.3390/ijms252413591. Int J Mol Sci. 2024. PMID: 39769353 Free PMC article.
-
Localization of nanospheres in pheochromocytoma-like cells following exposure to high-frequency electromagnetic fields at 18 GHz.R Soc Open Sci. 2022 Jun 29;9(6):220520. doi: 10.1098/rsos.220520. eCollection 2022 Jun. R Soc Open Sci. 2022. PMID: 35774138 Free PMC article.
References
-
- Bonetta, L. 2005. The inside scoop—evaluating gene delivery methods. Nat. Methods 2:875–883.
-
- DeBruin, K. A. , and Krassowska W.. 1998. Electroporation and shock‐induced transmembrane potential in a cardiac fiber during defibrillation strength shocks. Ann. Biomed. Eng. 26:584–596. - PubMed
-
- Dorsett, Y. , and Tuschi T.. 2004. siRNAs: applications in functional genomics and potential as therapeutics. Nat. Rev. Drug Discovery 3:318–329. - PubMed
-
- Duscher, D. , Barrera J., Wong V. W., Maan Z. N., Whittam A. J., Januszyk M., et al. 2015. Stem cells in wound healing: the future of regenerative medicine? A mini‐review. Gerontology. [Epub ahead of print] - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

