MicroCT angiography detects vascular formation and regression in skin wound healing
- PMID: 27009591
- PMCID: PMC4867264
- DOI: 10.1016/j.mvr.2016.03.006
MicroCT angiography detects vascular formation and regression in skin wound healing
Abstract
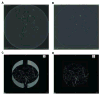
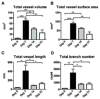
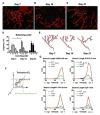
Properly regulated angiogenesis and arteriogenesis are essential for effective wound healing. Tissue injury induces robust new vessel formation and subsequent vessel maturation, which involves vessel regression and remodeling. Although formation of functional vasculature is essential for healing, alterations in vascular structure over the time course of skin wound healing are not well understood. Here, using high-resolution ex vivo X-ray micro-computed tomography (microCT), we describe the vascular network during healing of skin excisional wounds with highly detailed three-dimensional (3D) reconstructed images and associated quantitative analysis. We found that relative vessel volume, surface area and branching number are significantly decreased in wounds from day 7 to days 14 and 21. Segmentation and skeletonization analysis of selected branches from high-resolution images as small as 2.5μm voxel size show that branching orders are decreased in the wound vessels during healing. In histological analysis, we found that the contrast agent fills mainly arterioles, but not small capillaries nor large veins. In summary, high-resolution microCT revealed dynamic alterations of vessel structures during wound healing. This technique may be useful as a key tool in the study of the formation and regression of wound vessels.
Keywords: Angiogenesis; Angiography; Micro-computed tomography; Three-dimensional; Wound healing.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

