Decreased level of RASSF6 in sporadic colorectal cancer and its anti-tumor effects both in vitro and in vivo
- PMID: 27009808
- PMCID: PMC4991420
- DOI: 10.18632/oncotarget.7852
Decreased level of RASSF6 in sporadic colorectal cancer and its anti-tumor effects both in vitro and in vivo
Abstract
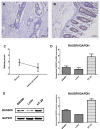
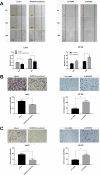
Ras-association domain family protein 6 (RASSF6) is a member of tumor suppressor RASSFs family with a wide range of function from RAS interaction, Hippo signaling involvement to cell cycle and apoptosis regulation. RASSF6 is reported inactivated in various types of cancer. However, whether RASSF6 is associated with colorectal cancer and the underlying mechanisms have yet to be investigated. In our previous exome sequencing study, we found a somatic loss-of-function (LoF) mutation in RASSF6 in one sporadic colorectal cancer (sCRC) patient, and two missense mutations in deep sequencing group of sCRC samples, implying the possibility that RASSF6 may be involved in the pathogenesis of sCRC. In this study, we demonstrate that RASSF6 acts as a tumor suppressor in colon cancer cells. Decreased level of RASSF6 was observed in adenocarcinoma compared to normal tissues, especially in advanced tumor cases. Further experiments showed exogenous introduction of RASSF6 into LoVo cells suppressed cell proliferation, migration, invasion, and induced apoptosis in vitro as well as tumor growth in vivo. In contrast, knockdown of RASSF6 in HT-29 cells showed the opposite effects. Taken together, our results suggest, in addition to epigenetics changes, functional somatic mutations may also contribute to the downregulation of RASSF6 and further participate in the pathogenesis of sCRC. RASSF6 may serve as a novel candidate against tumor growth for sCRC.
Keywords: RASSF6; apoptosis; loss-of-function mutation; proliferation; sporadic colorectal cancer.
Conflict of interest statement
We declared no conflicts of interest.
Figures


References
-
- Slattery ML. Diet, lifestyle, and colon cancer. Semin Gastrointest Dis. 2000;11:142–6. - PubMed
-
- Gonzalez-Gonzalez M, Garcia JG, Montero JA, Fernandez LM, Bengoechea O, Munez OB, Orfao A, Sayagues JM, Fuentes M. Genomics and proteomics approaches for biomarker discovery in sporadic colorectal cancer with metastasis. Cancer Genomics Proteomics. 2013;10:19–25. - PubMed
-
- Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nature Reviews Genetics. 2010;11:685–696. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

