Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study
- PMID: 27009916
- PMCID: PMC5099191
- DOI: 10.1136/annrheumdis-2015-208562
Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study
Abstract
Objectives: The efficacy and safety of sifalimumab were assessed in a phase IIb, randomised, double-blind, placebo-controlled study (NCT01283139) of adults with moderate to severe active systemic lupus erythematosus (SLE).
Methods: 431 patients were randomised and received monthly intravenous sifalimumab (200 mg, 600 mg or 1200 mg) or placebo in addition to standard-of-care medications. Patients were stratified by disease activity, interferon gene-signature test (high vs low based on the expression of four genes) and geographical region. The primary efficacy end point was the percentage of patients achieving an SLE responder index response at week 52.
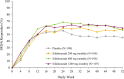
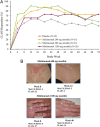
Results: Compared with placebo, a greater percentage of patients who received sifalimumab (all dosages) met the primary end point (placebo: 45.4%; 200 mg: 58.3%; 600 mg: 56.5%; 1200 mg 59.8%). Other improvements were seen in Cutaneous Lupus Erythematosus Disease Area and Severity Index score (200 mg and 1200 mg monthly), Physician's Global Assessment (600 mg and 1200 mg monthly), British Isles Lupus Assessment Group-based Composite Lupus Assessment (1200 mg monthly), 4-point reductions in the SLE Disease Activity Index-2000 score and reductions in counts of swollen joints and tender joints. Serious adverse events occurred in 17.6% of patients on placebo and 18.3% of patients on sifalimumab. Herpes zoster infections were more frequent with sifalimumab treatment.
Conclusions: Sifalimumab is a promising treatment for adults with SLE. Improvement was consistent across various clinical end points, including global and organ-specific measures of disease activity.
Trial registration number: NCT01283139; Results.
Keywords: Autoimmune Diseases; Systemic Lupus Erythematosus; Treatment.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
MK received a grant for this study from MedImmune/AstraZeneca; grants for other work from Bayer; and personal consultancy fees from INOVA Diagnostics, MedImmune, GlaxoSmithKline and UCB. JTM reports personal fees from MedImmune/AstraZeneca; and grants and personal fees from Genentech/Roche outside of the submitted work. VPW received personal fees from MedImmune/AstraZeneca, during the conduct of this study. RF, KK, GGI, JD, LW and WG received personal fees from AstraZeneca/MedImmune. WG and GGI hold stocks in AstraZeneca/MedImmune. JD has a patent pending. VPW is a named inventor and holds a patent for the CLASI copyright owned by the University of Pennsylvania. GGI, JD, LW and WG are employees of MedImmune.
Figures

Comment in
-
Connective tissue diseases: Targeting type I interferon in systemic lupus erythematosus.Nat Rev Rheumatol. 2016 Jul;12(7):377-8. doi: 10.1038/nrrheum.2016.83. Epub 2016 May 26. Nat Rev Rheumatol. 2016. PMID: 27225301 No abstract available.
-
Complex disease=complex trial? Lessons from a successful trial of anti-IFNα in SLE.Ann Rheum Dis. 2016 Nov;75(11):1899-1901. doi: 10.1136/annrheumdis-2016-209345. Epub 2016 Jun 21. Ann Rheum Dis. 2016. PMID: 27329393 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

