Chimeric Antigen Receptor T-Cell Therapy for the Community Oncologist
- PMID: 27009942
- PMCID: PMC4861363
- DOI: 10.1634/theoncologist.2015-0421
Chimeric Antigen Receptor T-Cell Therapy for the Community Oncologist
Abstract
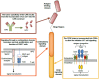
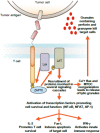
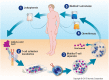
: The field of cancer immunotherapy has rapidly progressed in the past decade as several therapeutic modalities have entered into the clinic. One such immunotherapy that has shown promise in the treatment of cancer is the use of chimeric antigen receptor (CAR)-modified T lymphocytes. CARs are engineered receptors constructed from antigen recognition regions of antibodies fused to T-cell signaling and costimulatory domains that can be used to reprogram a patient's T cells to specifically target tumor cells. CAR T-cell therapy has demonstrated sustained complete responses for some patients with advanced leukemia, and a number of CAR therapies are being evaluated in clinical studies. CAR T-cell therapy-associated toxicities, including cytokine release syndrome, macrophage activation syndrome, and tumor lysis syndrome, have been observed and effectively managed in the clinic. In patients with significant clinical responses, sustained B-cell aplasia has also been observed and is a marker of CAR T-cell persistence that might provide long-term disease control. Education on CAR T-cell therapy efficacy and safety management is critical for clinicians and patients who are considering this novel type of treatment. In the present report, the current landscape of CAR T-cell therapy, the effective management of patients undergoing treatment, and which patients are the most suitable candidates for current trials are discussed.
Implications for practice: The present report describes the current status of chimeric antigen receptor (CAR) T lymphocytes as an immunotherapy for patients with relapsed or refractory B-cell malignancies. CAR T cells targeting CD19, a protein expressed on many B-cell malignancies, typically induce high complete response rates in patients with B-cell leukemia or lymphoma who have very limited therapeutic options. Recent clinical trial results of CD19 CAR T-cell therapies and the management of CAR T-cell-associated adverse events are discussed. The present report will therefore inform physicians regarding the efficacy and safety of CAR T cells as a therapy for B-cell malignancies.
Keywords: Antigen receptors; Immunotherapy; Leukemia; T lymphocytes.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. - PubMed
-
- DeBenedette MA, Shahinian A, Mak TW, et al. Costimulation of CD28- T lymphocytes by 4-1BB ligand. J Immunol. 1997;158:551–559. - PubMed
-
- Lu B, Finn OJ. T-cell death and cancer immune tolerance. Cell Death Differ. 2008;15:70–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

