Mutagenesis of Coronavirus nsp14 Reveals Its Potential Role in Modulation of the Innate Immune Response
- PMID: 27009949
- PMCID: PMC4934755
- DOI: 10.1128/JVI.03259-15
Mutagenesis of Coronavirus nsp14 Reveals Its Potential Role in Modulation of the Innate Immune Response
Abstract
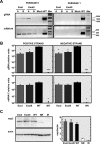
Coronavirus (CoV) nonstructural protein 14 (nsp14) is a 60-kDa protein encoded by the replicase gene that is part of the replication-transcription complex. It is a bifunctional enzyme bearing 3'-to-5' exoribonuclease (ExoN) and guanine-N7-methyltransferase (N7-MTase) activities. ExoN hydrolyzes single-stranded RNAs and double-stranded RNAs (dsRNAs) and is part of a proofreading system responsible for the high fidelity of CoV replication. nsp14 N7-MTase activity is required for viral mRNA cap synthesis and prevents the recognition of viral mRNAs as "non-self" by the host cell. In this work, a set of point mutants affecting different motifs within the ExoN domain of nsp14 was generated, using transmissible gastroenteritis virus as a model of Alphacoronavirus Mutants lacking ExoN activity were nonviable despite being competent in both viral RNA and protein synthesis. A specific mutation within zinc finger 1 (ZF-C) led to production of a viable virus with growth and viral RNA synthesis kinetics similar to that of the parental virus. Mutant recombinant transmissible gastroenteritis virus (TGEV) ZF-C (rTGEV-ZF-C) caused decreased cytopathic effect and apoptosis compared with the wild-type virus and reduced levels of dsRNA accumulation at late times postinfection. Consequently, the mutant triggered a reduced antiviral response, which was confirmed by evaluating different stages of the dsRNA-induced antiviral pathway. The expression of beta interferon (IFN-β), tumor necrosis factor (TNF), and interferon-stimulated genes in cells infected with mutant rTGEV-ZF-C was reduced compared to the levels seen with the parental virus. Overall, our data revealed a potential role for CoV nsp14 in modulation of the innate immune response.
Importance: The innate immune response is the first line of antiviral defense that culminates in the synthesis of interferon and proinflammatory cytokines to control viral replication. CoVs have evolved several mechanisms to counteract the innate immune response at different levels, but the role of CoV-encoded ribonucleases in preventing activation of the dsRNA-induced antiviral response has not been described to date. The introduction of a mutation in zinc finger 1 of the ExoN domain of nsp14 led to production of a virus that induced a weak antiviral response, most likely due to the accumulation of lower levels of dsRNA in the late phases of infection. These observations allowed us to propose a novel role for CoV nsp14 ExoN activity in counteracting the antiviral response, which could serve as a novel target for the design of antiviral strategies.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- de Groot RJ, Baker SC, Baric R, Enjuanes L, Gorbalenya AE, Holmes KV, Perlman S, Poon L, Rottier PJM, Talbot PJ, Woo PCY, Ziebuhr J. 2012. Coronaviridae, p 774–796. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy: ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA.
-
- de Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, Fouchier RA, Galiano M, Gorbalenya AE, Memish ZA, Perlman S, Poon LL, Snijder EJ, Stephens GM, Woo PC, Zaki AM, Zambon M, Ziebuhr J. 2013. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol 87:7790–7792. doi:10.1128/JVI.01244-13. - DOI - PMC - PubMed
-
- Enjuanes L, Gorbalenya AE, de Groot RJ, Cowley JA, Ziebuhr J, Snijder EJ. 2008. The Nidovirales, p 419–430. In Mahy BWJ, Van Regenmortel M, Walker P, Majumder-Russell D (ed), Encyclopedia of virology, 3rd ed Elsevier Ltd., Oxford, United Kingdom.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

