Hepatitis C virus NS5A protein cooperates with phosphatidylinositol 4-kinase IIIα to induce mitochondrial fragmentation
- PMID: 27010100
- PMCID: PMC4806301
- DOI: 10.1038/srep23464
Hepatitis C virus NS5A protein cooperates with phosphatidylinositol 4-kinase IIIα to induce mitochondrial fragmentation
Abstract
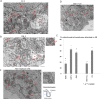
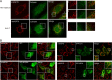
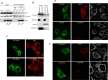
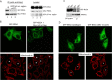
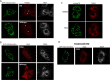
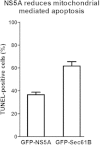
Hepatitis C virus (HCV) has long been observed to take advantage of the host mitochondria to support viral replication and assembly. The HCV core protein has been implicated to fragment host mitochondria. In this report, we have discovered that the non-structural protein 5A (NS5A) plays an instructive role in attaching ER with mitochondria, causing mitochondrial fragmentation. Dynamin-related protein 1(Drp1), a host protein essential to mitochondrial membrane fission, does not play a role in NS5A-induced mitochondrial fragmentation. Instead, phosphatidylinositol 4-kinase IIIα (PI4KA), which has been demonstrated to bind to NS5A and is required to support HCV life cycle, is required for NS5A to induce mitochondrial fragmentation. Both NS5A and core are required by HCV to fragment the mitochondria, as inhibiting either of their respective downstream proteins, PI4KA or Drp1, resulted in lengthening of mitochondria tubules in HCVcc-infected cells. By fragmenting the mitochondria, NS5A renders the cells more resistant to mitochondria mediated apoptosis. This finding indicates previously-ignored contribution of NS5A in HCV-induced mitochondria dysfunction.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

