Insulin-positive, Glut2-low cells present within mouse pancreas exhibit lineage plasticity and are enriched within extra-islet endocrine cell clusters
- PMID: 27010375
- PMCID: PMC4987018
- DOI: 10.1080/19382014.2016.1162367
Insulin-positive, Glut2-low cells present within mouse pancreas exhibit lineage plasticity and are enriched within extra-islet endocrine cell clusters
Abstract
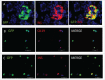
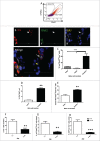
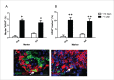
Regeneration of insulin-producing β-cells from resident pancreas progenitors requires an understanding of both progenitor identity and lineage plasticity. One model suggested that a rare β-cell sub-population within islets demonstrated multi-lineage plasticity. We hypothesized that β-cells from young mice (postnatal day 7, P7) exhibit such plasticity and used a model of islet dedifferentiation toward a ductal epithelial-cell phenotype to test this theory. RIPCre;Z/AP(+/+) mice were used to lineage trace the fate of β-cells during dedifferentiation culture by a human placental alkaline phosphatase (HPAP) reporter. There was a significant loss of HPAP-expressing β-cells in culture, but remaining HPAP(+) cells lost insulin expression while gaining expression of the epithelial duct cell marker cytokeratin-19 (Ck19). Flow cytometry and recovery of β-cell subpopulations from whole pancreas vs. islets suggest that the HPAP(+)Ck19(+) cells had derived from insulin-positive, glucose-transporter-2-low (Ins(+)Glut2(LO)) cells, representing 3.5% of all insulin-expressing cells. The majority of these cells were found outside of islets within clusters of <5 β-cells. These insulin(+)Glut2(LO) cells demonstrated a greater proliferation rate in vivo and in vitro as compared to insulin(+)Glut2(+) cells at P7, were retained into adulthood, and a subset differentiated into endocrine, ductal, and neural lineages, illustrating substantial plasticity. Results were confirmed using RIPCre;ROSA- eYFP mice. Quantitative PCR data indicated these cells possess an immature β-cell phenotype. These Ins(+)Glut2(LO) cells may represent a resident population of cells capable of forming new, functional β-cells, and which may be potentially exploited for regenerative therapies in the future.
Keywords: Glut2; differentiation; duct; islet; pancreas; plasticity; progenitor cell; β-cell.
Figures




References
-
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004; 429:41-6; PMID:15129273; http://dx.doi.org/10.1038/nature02520 - DOI - PubMed
-
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 2007; 12:817-26; PMID:17488631; http://dx.doi.org/10.1016/j.devcel.2007.04.011 - DOI - PubMed
-
- Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 2007; 117:2553-61; PMID:17786244; http://dx.doi.org/10.1172/JCI32959 - DOI - PMC - PubMed
-
- Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotech 2004; 22:1115-24; http://dx.doi.org/10.1038/nbt1004 - DOI - PubMed
-
- Smukler SR, Arntfield ME, Razavi R, Bikopoulos G, Karpowicz P, Seaberg R, Dai F, Lee S, Ahrens R, Fraser PE, et al.. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell 2011; 8:281-93; PMID:21362568; http://dx.doi.org/10.1016/j.stem.2011.01.015 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
