Health Professionals' Knowledge, Attitudes and Practices about Pharmacovigilance in India: A Systematic Review and Meta-Analysis
- PMID: 27010447
- PMCID: PMC4807086
- DOI: 10.1371/journal.pone.0152221
Health Professionals' Knowledge, Attitudes and Practices about Pharmacovigilance in India: A Systematic Review and Meta-Analysis
Abstract
Background: Spontaneous or voluntary reporting of suspected adverse drug reactions (ADRs) is one of the vital roles of all health professionals. In India, under-reporting of ADRs by health professionals is recognized as one of the leading causes of poor ADR signal detection. Therefore, reviewing the literature can provide a better understanding of the status of knowledge, attitude and practice (KAP) of Pharmacovigilance (PV) activities by health professionals.
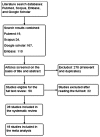
Methods: A systematic review was performed through Pubmed, Scopus, Embase and Google Scholar scientific databases. Studies pertaining to KAP of PV and ADR reporting by Indian health professionals between January 2011 and July 2015 were included in a meta-analysis.
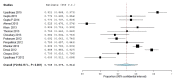
Results: A total of 28 studies were included in the systematic review and 18 of them were selected for meta-analysis. Overall, 55.6% (95% CI 44.4-66.9; p<0.001) of the population studied were not aware of the existence of the Pharmacovigilance Programme in India (PvPI), and 31.9% (95% CI 16.3-47.4; p<0.001) thought that "all drugs available in the market are safe". Furthermore, 28.7% (95% CI 16.4-40.9; p<0.001) of them were not interested in reporting ADRs and 74.5%, (95% CI 67.9-81.9; p<0.001) never reported any ADR to PV centers.
Conclusion: There was an enormous gap of KAP towards PV and ADR reporting, particularly PV practice in India. There is therefore an urgent need for educational awareness, simplification of the ADR reporting process, and implementation of imperative measures to practice PV among healthcare professionals. In order to understand the PV status, PvPI should procedurally assess the KAP of health professionals PV activities in India.
Conflict of interest statement
Figures





References
-
- Sengupta A. Universal Health care in India making it public. Ontario, Canada: IRDC; 2013. May p. 23. Report No.: 19.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

