Leucine induced dephosphorylation of Sestrin2 promotes mTORC1 activation
- PMID: 27010498
- PMCID: PMC4899281
- DOI: 10.1016/j.cellsig.2016.03.008
Leucine induced dephosphorylation of Sestrin2 promotes mTORC1 activation
Abstract
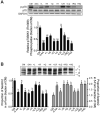
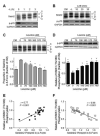
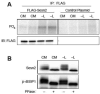
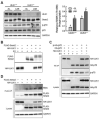
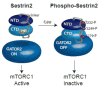
The studies described herein were designed to explore the role of Sestrin2 in mediating the selective action of leucine to activate mTORC1. The results demonstrate that Sestrin2 is a phosphoprotein and that its phosphorylation state is responsive to the availability of leucine, but not other essential amino acids. Moreover, leucine availability-induced alterations in Sestrin2 phosphorylation correlated temporally and dose dependently with the activation state of mTORC1, there being a reciprocal relationship between the degree of phosphorylation of Sestrin2 and the extent of repression of mTORC1. With leucine deprivation, Sestrin2 became more highly phosphorylated and interacted more strongly with proteins of the GATOR2 complex. Notably, in cells lacking the protein kinase ULK1, the activation state of mTORC1 was elevated in leucine-deficient medium, such that the effect of re-addition of the amino acid was blunted. In contrast, overexpression of ULK1 led to hyperphosphorylation of Sestrin2 and enhanced its interaction with GATOR2. Neither rapamycin nor Torin2 had any effect on Sestrin2 phosphorylation, suggesting that leucine deprivation-induced repression of mTORC1 was not responsible for the action of ULK1 on Sestrin2. Mass spectrometry analysis of Sestrin2 revealed three phosphorylation sites that are conserved across mammalian species. Mutation of the three sites to phospho-mimetic amino acids in exogenously expressed Sestrin2 promoted its interaction with GATOR2 and dramatically repressed mTORC1 even in the presence of leucine. Overall, the results support a model in which leucine selectively promotes dephosphorylation of Sestrin2, causing it to dissociate from and thereby activate GATOR2, leading to activation of mTORC1.
Keywords: Amino acids; Mechanistic target of rapamycin; Rag GTPases; Signaling; ULK1.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


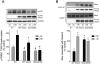
Similar articles
-
Sestrin2 is a leucine sensor for the mTORC1 pathway.Science. 2016 Jan 1;351(6268):43-8. doi: 10.1126/science.aab2674. Epub 2015 Oct 8. Science. 2016. PMID: 26449471 Free PMC article.
-
Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway.Science. 2016 Jan 1;351(6268):53-8. doi: 10.1126/science.aad2087. Epub 2015 Nov 19. Science. 2016. PMID: 26586190 Free PMC article.
-
GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2.Genes Dev. 2015 Nov 15;29(22):2331-6. doi: 10.1101/gad.269324.115. Epub 2015 Nov 5. Genes Dev. 2015. PMID: 26543160 Free PMC article.
-
Leucine and mTORC1: a complex relationship.Am J Physiol Endocrinol Metab. 2012 Jun 1;302(11):E1329-42. doi: 10.1152/ajpendo.00525.2011. Epub 2012 Feb 21. Am J Physiol Endocrinol Metab. 2012. PMID: 22354780 Review.
-
Key mediators of intracellular amino acids signaling to mTORC1 activation.Amino Acids. 2015 May;47(5):857-67. doi: 10.1007/s00726-015-1937-x. Epub 2015 Feb 21. Amino Acids. 2015. PMID: 25701492 Review.
Cited by
-
Resistance exercise enhances long-term mTORC1 sensitivity to leucine.Mol Metab. 2022 Dec;66:101615. doi: 10.1016/j.molmet.2022.101615. Epub 2022 Oct 14. Mol Metab. 2022. PMID: 36252815 Free PMC article.
-
Estrogen receptor alpha mediates 17β-estradiol, up-regulates autophagy and alleviates hydrogen peroxide-induced vascular senescence.Biogerontology. 2023 Oct;24(5):783-799. doi: 10.1007/s10522-023-10015-4. Epub 2023 Jan 23. Biogerontology. 2023. PMID: 36683095
-
The functions and roles of sestrins in regulating human diseases.Cell Mol Biol Lett. 2022 Jan 3;27(1):2. doi: 10.1186/s11658-021-00302-8. Cell Mol Biol Lett. 2022. PMID: 34979914 Free PMC article. Review.
-
Sesn-1 is required for lifespan extension during caloric deprivation in C. elegans through inhibition of mTORC1 and activation of autophagy.Aging (Albany NY). 2025 Jul 28;17(7):1834-1851. doi: 10.18632/aging.206290. Epub 2025 Jul 28. Aging (Albany NY). 2025. PMID: 40728520 Free PMC article.
-
Evidence for a role for Sestrin1 in mediating leucine-induced activation of mTORC1 in skeletal muscle.Am J Physiol Endocrinol Metab. 2019 May 1;316(5):E817-E828. doi: 10.1152/ajpendo.00522.2018. Epub 2019 Mar 5. Am J Physiol Endocrinol Metab. 2019. PMID: 30835510 Free PMC article.
References
-
- Fulks RM, Li JB, Goldberg AL. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975;250:290–298. - PubMed
-
- Li JB, Jefferson LS. Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochem Biophys Acta. 1978;544:351–359. - PubMed
-
- Morgan HE, Earl DC, Broadus A, Wolpert EB, Giger KE, Jefferson LS. Regulation of protein synthesis in heart muscle. I. Effect of amino acid levels on protein synthesis. J Biol Chem. 1971;246:2152–2162. - PubMed
-
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of post-absorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

