CETSA screening identifies known and novel thymidylate synthase inhibitors and slow intracellular activation of 5-fluorouracil
- PMID: 27010513
- PMCID: PMC4820820
- DOI: 10.1038/ncomms11040
CETSA screening identifies known and novel thymidylate synthase inhibitors and slow intracellular activation of 5-fluorouracil
Abstract
Target engagement is a critical factor for therapeutic efficacy. Assessment of compound binding to native target proteins in live cells is therefore highly desirable in all stages of drug discovery. We report here the first compound library screen based on biophysical measurements of intracellular target binding, exemplified by human thymidylate synthase (TS). The screen selected accurately for all the tested known drugs acting on TS. We also identified TS inhibitors with novel chemistry and marketed drugs that were not previously known to target TS, including the DNA methyltransferase inhibitor decitabine. By following the cellular uptake and enzymatic conversion of known drugs we correlated the appearance of active metabolites over time with intracellular target engagement. These data distinguished a much slower activation of 5-fluorouracil when compared with nucleoside-based drugs. The approach establishes efficient means to associate drug uptake and activation with target binding during drug discovery.
Conflict of interest statement
D.M.M. and P.N. are founders of the company Pelago Bioscience AB. D.C., A.L., A.M., P.A, R.J., T.L., H.Al., M.H. and H.Ax. declare no competing financial interests.
Figures
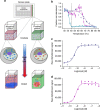


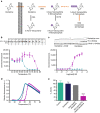
References
-
- Morgan P. et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving Phase II survival. Drug Discov. Today 17, 419–424 (2012). - PubMed
-
- Bunnage M. E., Chekler E. L. P. & Jones L. H. Target validation using chemical probes. Nat. Chem. Biol. 9, 195–199 (2013). - PubMed
-
- Cook D. et al. Lessons learned from the fate of AstraZeneca's drug pipeline: a five-dimensional framework. Nat. Rev. Drug Discov. 13, 419–431 (2014). - PubMed
-
- Durham T. B. & Blanco M.-J. Target engagement in lead generation. Bioorg. Med. Chem. Lett. 25, 998–1008 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

