Tau pathology-mediated presynaptic dysfunction
- PMID: 27012611
- PMCID: PMC4887082
- DOI: 10.1016/j.neuroscience.2016.03.044
Tau pathology-mediated presynaptic dysfunction
Abstract
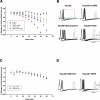
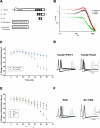
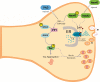
Brain tauopathies are characterized by abnormal processing of tau protein. While somatodendritic tau mislocalization has attracted considerable attention in tauopathies, the role of tau pathology in axonal transport, connectivity and related dysfunctions remains obscure. We have previously shown using the squid giant synapse that presynaptic microinjection of recombinant human tau protein (htau42) results in failure of synaptic transmission. Here, we evaluated molecular mechanisms mediating this effect. Thus, the initial event, observed after htau42 presynaptic injection, was an increase in transmitter release. This event was mediated by calcium release from intracellular stores and was followed by a reduction in evoked transmitter release. The effect of htau42 on synaptic transmission was recapitulated by a peptide comprising the phosphatase-activating domain of tau, suggesting activation of phosphotransferases. Accordingly, findings indicated that htau42-mediated toxicity involves the activities of both GSK3 and Cdk5 kinases.
Keywords: Cdk5; GSK3; IP3 receptor; phosphatase-activating domain of tau; ryanodine receptor; tauopathy.
Copyright © 2016 IBRO. All rights reserved.
Figures
References
-
- Cash AD, Aliev G, Siedlak SL, Nunomura A, Fujioka H, Zhu X, Raina AK, Vinters HV, Tabaton M, Johnson AB, Paula-Barbosa M, Avila J, Jones PK, Castellani RJ, Smith MA, Perry G. Microtubule reduction in Alzheimer's disease and aging is independent of tau filament formation. Am J Pathol. 2003;162:1623–1627. - PMC - PubMed
-
- Collin T, Marty A, Llano I. Presynaptic calcium stores and synaptic transmission. Curr Opin Neurobiol. 2005;15:275–281. - PubMed
References for Table 1
-
- Ma HT, et al. Assessment of the role of the inositol 1,4,5-trisphosphate receptor in the activation of transient receptor potential channels and store-operated Ca2+ entry channels. J Biol Chem. 2001;276(22):18888–96. - PubMed
-
- Maruyama T, et al. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122(3):498–505. - PubMed
-
- Missiaen L, et al. 2-Aminoethoxydiphenyl borate affects the inositol 1,4,5-trisphosphate receptor, the intracellular Ca2+ pump and the non-specific Ca2+ leak from the non-mitochondrial Ca2+ stores in permeabilized A7r5 cells. Cell Calcium. 2001;29(2):111–6. - PubMed
-
- Gafni J, et al. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19(3):723–33. - PubMed
-
- De Smet P, et al. Xestospongin C is an equally potent inhibitor of the inositol 1,4,5-trisphosphate receptor and the endoplasmic-reticulum Ca(2+) pumps. Cell Calcium. 1999;26(1-2):9–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

