Allosteric heat shock protein 70 inhibitors block hepatitis C virus assembly
- PMID: 27013001
- PMCID: PMC4833571
- DOI: 10.1016/j.ijantimicag.2016.01.012
Allosteric heat shock protein 70 inhibitors block hepatitis C virus assembly
Abstract
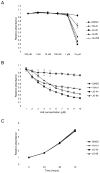
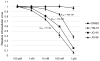
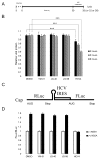
The human molecular chaperones heat shock protein 70 (Hsp70) and heat shock cognate protein 70 (Hsc70) bind to the hepatitis C viral nonstructural protein 5A (NS5A) and regulate its activity. Specifically, Hsp70 is involved in NS5A-augmented internal ribosomal entry site (IRES)-mediated translation of the viral genome, whilst Hsc70 appears to be primarily important for intracellular infectious virion assembly. To better understand the importance of these two chaperones in the viral life cycle, infected human cells were treated with allosteric Hsp70/Hsc70 inhibitors (AHIs). Treatment with AHIs significantly reduced the production of intracellular virus at concentrations that were non-toxic to human hepatoma Huh7.5 cells. The supernatant of treated cultures was then used to infect naïve cells, revealing that AHIs also lowered levels of secreted virus. In contrast to their effects on virion assembly, AHIs did not impact the stability of NS5A or viral protein translation in IRES assays. These results suggest that Hsc70 plays a particularly important and sensitive role in virion assembly. Indeed, it was found that combination of AHIs with a peptide-based viral translation inhibitor exhibited additive antiviral activity. Together these results suggest that the host Hsc70 is a new antiviral target and that its inhibitors utilise a new mechanism of action.
Keywords: Allosteric heat shock protein inhibitors; Hepatitis C virus; Hsc70; Hsp70; Viral assembly; Viral translation.
Copyright © 2016 Elsevier B.V. and the International Society of Chemotherapy. All rights reserved.
Conflict of interest statement
Figures




References
-
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–67. - PubMed
-
- El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35(5 Suppl 2):S72–8. - PubMed
-
- Donaldson EF, Harrington PR, O’Rear JJ, Naeger LK. Clinical evidence and bioinformatics characterization of potential hepatitis C virus resistance pathways for sofosbuvir. Hepatology. 2015;61:56–65. - PubMed
-
- Shiffman ML. What future for ribavirin? Liver Int. 2009;29(Suppl 1):68–73. - PubMed
-
- Hedskog C, Doehle B, Chodavarapu K, Gontcharova V, Crespo Garcia J, De Knegt R, et al. Characterization of hepatitis C virus intergenotypic recombinant strains and associated virological response to sofosbuvir/ribavirin. Hepatology. 2015;61:471–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

