State-of-the-art Imaging of Pancreatic Neuroendocrine Tumors
- PMID: 27013371
- PMCID: PMC4808582
- DOI: 10.1016/j.soc.2015.11.007
State-of-the-art Imaging of Pancreatic Neuroendocrine Tumors
Abstract
Pancreatic neuroendocrine tumors are rare tumors that present many imaging challenges, from detecting small functional tumors to fully staging large nonfunctioning tumors, including identifying all sites of metastatic disease, particularly nodal and hepatic, and depicting vascular involvement. The correct choice of imaging modality requires knowledge of the tumor type (eg, gastrinoma versus insulinoma), and also the histology (well vs poorly differentiated). Evolving techniques in computed tomography (CT), MRI, endoscopic ultrasonography, and nuclear medicine, such as dual-energy CT, diffusion-weighted MRI, liver-specific magnetic resonance contrast agents, and new nuclear medicine agents, offer new ways to visualize, and ultimately manage, these tumors.
Keywords: Computed tomography; Dual energy; Endoscopic ultrasonography; Imaging; MRI; Octreotide; PET/CT; Pancreatic neuroendocrine tumor.
Copyright © 2016 Elsevier Inc. All rights reserved.
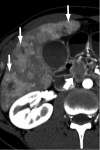
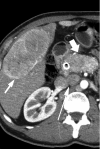
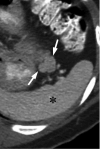
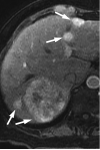
Figures















































References
-
- Fraenkel M, Kim MK, Faggiano A, et al. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol. 2012;26(6):691–703. - PubMed
-
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40(1):1–18. vii. - PubMed
-
- Zhou J, Enewold L, Stojadinovic A, et al. Incidence rates of exocrine and endocrine pancreatic cancers in the United States. Cancer Causes Control. 2010;21(6):853–861. - PubMed
-
- Baur AD, Pavel M, Prasad V, et al. Acta radiologica (Stockholm, Sweden : 1987) 2015. Diagnostic imaging of pancreatic neuroendocrine neoplasms (pNEN): tumor detection, staging, prognosis, and response to treatment. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

