Receptor Activity-modifying Proteins 2 and 3 Generate Adrenomedullin Receptor Subtypes with Distinct Molecular Properties
- PMID: 27013657
- PMCID: PMC4882435
- DOI: 10.1074/jbc.M115.688218
Receptor Activity-modifying Proteins 2 and 3 Generate Adrenomedullin Receptor Subtypes with Distinct Molecular Properties
Abstract
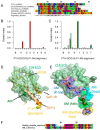
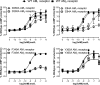
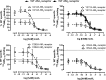
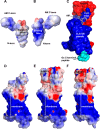
Adrenomedullin (AM) is a peptide hormone with numerous effects in the vascular systems. AM signals through the AM1 and AM2 receptors formed by the obligate heterodimerization of a G protein-coupled receptor, the calcitonin receptor-like receptor (CLR), and receptor activity-modifying proteins 2 and 3 (RAMP2 and RAMP3), respectively. These different CLR-RAMP interactions yield discrete receptor pharmacology and physiological effects. The effective design of therapeutics that target the individual AM receptors is dependent on understanding the molecular details of the effects of RAMPs on CLR. To understand the role of RAMP2 and -3 on the activation and conformation of the CLR subunit of AM receptors, we mutated 68 individual amino acids in the juxtamembrane region of CLR, a key region for activation of AM receptors, and determined the effects on cAMP signaling. Sixteen CLR mutations had differential effects between the AM1 and AM2 receptors. Accompanying this, independent molecular modeling of the full-length AM-bound AM1 and AM2 receptors predicted differences in the binding pocket and differences in the electrostatic potential of the two AM receptors. Druggability analysis indicated unique features that could be used to develop selective small molecule ligands for each receptor. The interaction of RAMP2 or RAMP3 with CLR induces conformational variation in the juxtamembrane region, yielding distinct binding pockets, probably via an allosteric mechanism. These subtype-specific differences have implications for the design of therapeutics aimed at specific AM receptors and for understanding the mechanisms by which accessory proteins affect G protein-coupled receptor function.
Keywords: G protein-coupled receptor (GPCR); GPCR; RAMP; adrenomedullin; allosteric regulation; cardiovascular disease; conformational change; extracellular loops; molecular modeling; receptor activity-modifying protein.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






References
-
- Nishikimi T., Kuwahara K., Nakagawa Y., Kangawa K., and Nakao K. (2013) Adrenomedullin in cardiovascular disease: a useful biomarker, its pathological roles and therapeutic application. Curr. Protein Pept. Sci. 14, 256–267 - PubMed
-
- Manuela C., Laura S., Benedetta S., Raffaele C., Alessandro V., Chiara C., Tommaso P., Daniela G., and Silvia D. R. (2014) Adrenomedullin and intermedin gene transcription is increased in leukocytes of patients with chronic heart failure at different stages of the disease. Peptides 55, 13–16 - PubMed
-
- Burley D. S., Hamid S. A., and Baxter G. F. (2007) Cardioprotective actions of peptide hormones in myocardial ischemia. Heart Fail. Rev. 12, 279–291 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

