Structure and Metal Binding Properties of a Poxvirus Resolvase
- PMID: 27013661
- PMCID: PMC4900259
- DOI: 10.1074/jbc.M115.709139
Structure and Metal Binding Properties of a Poxvirus Resolvase
Abstract
Poxviruses replicate their linear genomes by forming concatemers that must be resolved into monomeric units to produce new virions. A viral resolvase cleaves DNA four-way junctions extruded at the concatemer junctions to produce monomeric genomes. This cleavage reaction is required for viral replication, so the resolvase is an attractive target for small molecule inhibitors. To provide a platform for understanding resolvase mechanism and designing inhibitors, we have determined the crystal structure of the canarypox virus (CPV) resolvase. CPV resolvase is dimer of RNase H superfamily domains related to Escherichia coli RuvC, with an active site lined by highly conserved acidic residues that bind metal ions. There are several intriguing structural differences between resolvase and RuvC, and a model of the CPV resolvase·Holliday junction complex provides insights into the consequences of these differences, including a plausible explanation for the weak sequence specificity exhibited by the poxvirus enzymes. The model also explains why the poxvirus resolvases are more promiscuous than RuvC, cleaving a variety of branched, bulged, and flap-containing substrates. Based on the unique active site structure observed for CPV resolvase, we have carried out a series of experiments to test divalent ion usage and preferences. We find that the two resolvase metal binding sites have different preferences for Mg(2+) versus Mn(2+) Optimal resolvase activity is maintained with 5 μm Mn(2+) and 100 μm Mg(2+), concentrations that are well below those required for either metal alone. Together, our findings provide biochemical insights and structural models that will facilitate studying poxvirus replication and the search for efficient poxvirus inhibitors.
Keywords: Holliday junction; enzyme structure; metalloenzyme; poxvirus; resolvase; viral replication.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




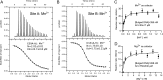
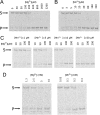

References
-
- Moss B. (2001) Poxviridae: the viruses and their replication. In Virology (Fields B. N., ed), pp. 2637–2672, Lippincott-Raven, Philadelphia, PA
-
- Moss B. (1991) Vaccinia virus: a tool for research and vaccine development. Science 252, 1662–1667 - PubMed
-
- Merchlinsky M., Garon C. F., and Moss B. (1988) Molecular cloning and sequence of the concatemer junction from vaccinia virus replicative DNA. Viral nuclease cleavage sites in cruciform structures. J. Mol. Biol. 199, 399–413 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

