Experience Affects Critical Period Plasticity in the Visual Cortex through an Epigenetic Regulation of Histone Post-Translational Modifications
- PMID: 27013673
- PMCID: PMC6601734
- DOI: 10.1523/JNEUROSCI.1787-15.2016
Experience Affects Critical Period Plasticity in the Visual Cortex through an Epigenetic Regulation of Histone Post-Translational Modifications
Abstract
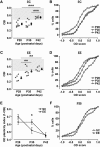
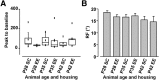
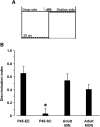
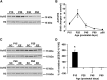
During an early phase of enhanced sensitivity called the critical period (CP), monocular deprivation causes a shift in the response of visual cortex binocular neurons in favor of the nondeprived eye, a process named ocular dominance (OD) plasticity. While the time course of the CP for OD plasticity can be modulated by genetic/pharmacological interventions targeting GABAergic inhibition, whether an increased sensory-motor experience can affect this major plastic phenomenon is not known. We report that exposure to environmental enrichment (EE) accelerated the closure of the CP for OD plasticity in the rat visual cortex. Histone H3 acetylation was developmentally regulated in primary visual cortex, with enhanced levels being detectable early in enriched pups, and chromatin immunoprecipitation revealed an increase at the level of the BDNF P3 promoter. Administration of the histone deacetylase inhibitor SAHA (suberoylanilide hydroxamic acid) to animals reared in a standard cage mimicked the increase in H3 acetylation observed in the visual cortex and resulted in an accelerated decay of OD plasticity. Finally, exposure to EE in adulthood upregulated H3 acetylation and was paralleled by a reopening of the CP. These findings demonstrate a critical involvement of the epigenetic machinery as a mediator of visual cortex developmental plasticity and of the impact of EE on OD plasticity.
Significance statement: While it is known that an epigenetic remodeling of chromatin structure controls developmental plasticity in the visual cortex, three main questions have remained open. Which is the physiological time course of histone modifications? Is it possible, by manipulating the chromatin epigenetic state, to modulate plasticity levels during the critical period? How can we regulate histone acetylation in the adult brain in a noninvasive manner? We show that the early exposure of rat pups to enriching environmental conditions accelerates the critical period for plasticity in the primary visual cortex, linking this effect to increased histone acetylation, specifically at the BDNF gene level. Moreover, we report that the exposure of adult animals to environmental enrichment enhances histone acetylation and reopens juvenile-like plasticity.
Keywords: BDNF; critical period; histone acetylation; ocular dominance plasticity.
Copyright © 2016 the authors 0270-6474/16/363430-11$15.00/0.
Figures



References
-
- Baroncelli L, Bonaccorsi J, Milanese M, Bonifacino T, Giribaldi F, Manno I, Cenni MC, Berardi N, Bonanno G, Maffei L, Sale A. Enriched experience and recovery from amblyopia in adult rats: impact of motor, social and sensory components. Neuropharmacology. 2012;62:2388–2397. doi: 10.1016/j.neuropharm.2012.02.010. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
