Dysfunctional Calcium and Glutamate Signaling in Striatal Astrocytes from Huntington's Disease Model Mice
- PMID: 27013675
- PMCID: PMC4804005
- DOI: 10.1523/JNEUROSCI.3693-15.2016
Dysfunctional Calcium and Glutamate Signaling in Striatal Astrocytes from Huntington's Disease Model Mice
Abstract
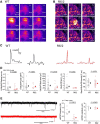
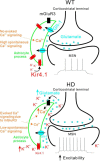
Astrocytes tile the entire CNS, but their functions within neural circuits in health and disease remain incompletely understood. We used genetically encoded Ca(2+)and glutamate indicators to explore the rules for astrocyte engagement in the corticostriatal circuit of adult wild-type (WT) and Huntington's disease (HD) model mice at ages not accompanied by overt astrogliosis (at approximately postnatal days 70-80). WT striatal astrocytes displayed extensive spontaneous Ca(2+)signals, but did not respond to cortical stimulation, implying that astrocytes were largely disengaged from cortical input in healthy tissue. In contrast, in HD model mice, spontaneous Ca(2+)signals were significantly reduced in frequency, duration, and amplitude, but astrocytes responded robustly to cortical stimulation with evoked Ca(2+)signals. These action-potential-dependent astrocyte Ca(2+)signals were mediated by neuronal glutamate release during cortical stimulation, accompanied by prolonged extracellular glutamate levels near astrocytes and tightly gated by Glt1 glutamate transporters. Moreover, dysfunctional Ca(2+)and glutamate signaling that was observed in HD model mice was largely, but not completely, rescued by astrocyte specific restoration of Kir4.1, emphasizing the important contributions of K(+)homeostatic mechanisms that are known to be reduced in HD model mice. Overall, our data show that astrocyte engagement in the corticostriatal circuit is markedly altered in HD. Such prodromal astrocyte dysfunctions may represent novel therapeutic targets in HD and other brain disorders.
Significance statement: We report how early-onset astrocyte dysfunction without detectable astrogliosis drives disease-related processes in a mouse model of Huntington's disease (HD). The cellular mechanisms involve astrocyte homeostasis and signaling mediated by Kir4.1, Glt1, and Ca(2+) The data show that the rules for astrocyte engagement in a neuronal circuit are fundamentally altered in a brain disease caused by a known molecular defect and that fixing early homeostasis dysfunction remedies additional cellular deficits. Overall, our data suggest that key aspects of altered striatal function associated with HD may be triggered, at least in part, by dysfunctional astrocytes, thereby providing details of an emerging striatal microcircuit mechanism in HD. Such prodromal changes in astrocytes may represent novel therapeutic targets.
Keywords: GCaMP; Huntington's disease; astrocyte; calcium.
Copyright © 2016 the authors 0270-6474/16/363453-18$15.00/0.
Figures

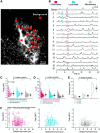
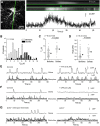

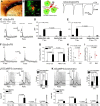

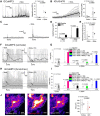

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
