A cross-sectional study to assess inhalation device handling and patient satisfaction in COPD
- PMID: 27013871
- PMCID: PMC4777273
- DOI: 10.2147/COPD.S91118
A cross-sectional study to assess inhalation device handling and patient satisfaction in COPD
Abstract
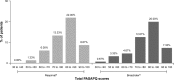
Delivery of inhaled medications via an inhaler device underpins the effectiveness of treatment for patients with chronic obstructive pulmonary disease (COPD). Correct inhaler technique among patients is also a predictor of achieving treatment compliance and adherence. Reporting of patient satisfaction with inhalers is therefore gaining increasing attention and is now recognized as an important patient-reported outcome in clinical trials involving patients with COPD or asthma. In this cross-sectional study, we use the validated Patient Satisfaction and Preference Questionnaire (PASAPQ) to assess the handling and satisfaction for Respimat(®) Soft Mist™ Inhaler (SMI) compared with the Breezhaler(®) dry powder inhaler (DPI) among patients with COPD in Spain. Patients were already assigned to therapy with either SPIRIVA(®) (tiotropium) Respimat(®) or with Hirobriz(®)/Onbrez(®)/Oslif(®) (indacaterol) Breezhaler(®) for at least 3 but not more than 6 months before completing the PASAPQ at a single visit to the study site. The primary endpoint of the trial was the mean total PASAPQ score. Secondary endpoints were the performance score domain of the PASAPQ, the convenience score domain of the PASAPQ, and the overall satisfaction score of the PASAPQ. For the primary endpoint, the mean PASAPQ total score in the Respimat(®) and Breezhaler(®) groups was 80.7 and 79.9, respectively (difference of 0.8, 95% confidence interval [CI] -2.9 to 4.5; P=0.67). The mean total performance scores were 82.5 and 78.2 (difference of 4.3, 95% CI -0.3 to 8.9; P=0.06), and the mean total convenience scores were 78.6 and 81.9 (difference of -3.3, 95% CI -7.0 to 0.4; P=0.08) for the Respimat(®) and Breezhaler(®) groups, respectively. Patients gave the Respimat(®) SMI and the Breezhaler(®) DPI overall satisfaction PASAPQ scores of 6.0 and 5.9, respectively, which shows that patients were satisfied with these inhalers.
Keywords: Breezhaler®; COPD; Respimat®; handling; inhaler.
Figures
References
-
- Moroni-Zentgraf P. Impact of patient needs on design and usage of an inhalation device in respiratory medicine. Respir Drug Deliv Eur. 2013;1:141–152.
-
- Virchow JC, Crompton GK, Dal NR, et al. Importance of inhaler devices in the management of airway disease. Respir Med. 2008;102(1):10–19. - PubMed
-
- Lavorini F, Magnan A, Dubus JC, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med. 2008;102(4):593–604. - PubMed
-
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. - PubMed
-
- Molimard M. How to achieve good compliance and adherence with inhalation therapy. Curr Med Res Opin. 2005;21(Suppl 4):S33–S37. - PubMed