KIF5C S176 Phosphorylation Regulates Microtubule Binding and Transport Efficiency in Mammalian Neurons
- PMID: 27013971
- PMCID: PMC4791394
- DOI: 10.3389/fncel.2016.00057
KIF5C S176 Phosphorylation Regulates Microtubule Binding and Transport Efficiency in Mammalian Neurons
Abstract
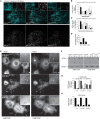
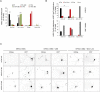
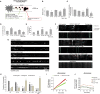
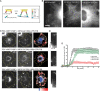
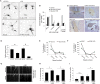
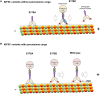
Increased phosphorylation of the KIF5 anterograde motor is associated with impaired axonal transport and neurodegeneration, but paradoxically also with normal transport, though the details are not fully defined. JNK phosphorylates KIF5C on S176 in the motor domain; a site that we show is phosphorylated in brain. Microtubule pelleting assays demonstrate that phosphomimetic KIF5C(1-560)(S176D) associates weakly with microtubules compared to KIF5C(1-560)(WT). Consistent with this, 50% of KIF5C(1-560)(S176D) shows diffuse movement in neurons. However, the remaining 50% remains microtubule bound and displays decreased pausing and increased bidirectional movement. The same directionality switching is observed with KIF5C(1-560)(WT) in the presence of an active JNK chimera, MKK7-JNK. Yet, in cargo trafficking assays where peroxisome cargo is bound, KIF5C(1-560)(S176D)-GFP-FRB transports normally to microtubule plus ends. We also find that JNK increases the ATP hydrolysis of KIF5C in vitro. These data suggest that phosphorylation of KIF5C-S176 primes the motor to either disengage entirely from microtubule tracks as previously observed in response to stress, or to display improved efficiency. The final outcome may depend on cargo load and motor ensembles.
Keywords: BDNF; JNK; SCG10; axonal transport; kinesin; molecular motors; neurons; phosphorylation.
Figures

References
-
- Björkblom B., Ostman N., Hongisto V., Komarovski V., Filén J. J., Nyman T. A., et al. (2005). Constitutively active cytoplasmic c-Jun N-terminal kinase 1 is a dominant regulator of dendritic architecture: role of microtubule-associated protein 2 as an effector. J. Neurosci. 25, 6350–6361. 10.1523/JNEUROSCI.1517-05.2005 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

