Targeting Treatment-Resistant Auditory Verbal Hallucinations in Schizophrenia with fMRI-Based Neurofeedback - Exploring Different Cases of Schizophrenia
- PMID: 27014102
- PMCID: PMC4791600
- DOI: 10.3389/fpsyt.2016.00037
Targeting Treatment-Resistant Auditory Verbal Hallucinations in Schizophrenia with fMRI-Based Neurofeedback - Exploring Different Cases of Schizophrenia
Abstract
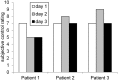
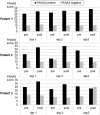
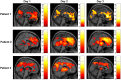
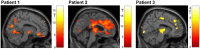
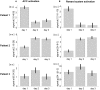
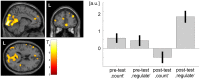
Auditory verbal hallucinations (AVHs) are a hallmark of schizophrenia and can significantly impair patients' emotional, social, and occupational functioning. Despite progress in psychopharmacology, over 25% of schizophrenia patients suffer from treatment-resistant hallucinations. In the search for alternative treatment methods, neurofeedback (NF) emerges as a promising therapy tool. NF based on real-time functional magnetic resonance imaging (rt-fMRI) allows voluntarily change of the activity in a selected brain region - even in patients with schizophrenia. This study explored effects of NF on ongoing AVHs. The selected participants were trained in the self-regulation of activity in the anterior cingulate cortex (ACC), a key monitoring region involved in generation and intensity modulation of AVHs. Using rt-fMRI, three right-handed patients, suffering from schizophrenia and ongoing, treatment-resistant AVHs, learned control over ACC activity on three separate days. The effect of NF training on hallucinations' severity was assessed with the Auditory Vocal Hallucination Rating Scale (AVHRS) and on the affective state - with the Positive and Negative Affect Schedule (PANAS). All patients yielded significant upregulation of the ACC and reported subjective improvement in some aspects of AVHs (AVHRS) such as disturbance and suffering from the voices. In general, mood (PANAS) improved during NF training, though two patients reported worse mood after NF on the third day. ACC and reward system activity during NF learning and specific effects on mood and symptoms varied across the participants. None of them profited from the last training set in the prolonged three-session training. Moreover, individual differences emerged in brain networks activated with NF and in symptom changes, which were related to the patients' symptomatology and disease history. NF based on rt-fMRI seems a promising tool in therapy of AVHs. The patients, who suffered from continuous hallucinations for years, experienced symptom changes that may be attributed to the NF training. In order to assess the effectiveness of NF as a therapeutic method, this effect has to be studied systematically in larger groups; further, long-term effects need to be assessed. Particularly in schizophrenia, future NF studies should take into account the individual differences in reward processing, fatigue, and motivation to develop individualized training protocols.
Keywords: affect; anterior cingulate cortex; auditory hallucinations; brain–computer interface; functional magnetic resonance imaging; neurofeedback; schizophrenia; self-regulation.
Figures

References
-
- Sartorius N, Jablensky A, Korten A, Ernberg G, Anker M, Cooper JE, et al. Early manifestations and first-contact incidence of schizophrenia in different cultures. A preliminary report on the initial evaluation phase of the WHO Collaborative Study on determinants of outcome of severe mental disorders. Psychol Med (1986) 16:909–28. 10.1017/S0033291700011910 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

