How Can We Study the Evolution of Animal Minds?
- PMID: 27014163
- PMCID: PMC4791388
- DOI: 10.3389/fpsyg.2016.00358
How Can We Study the Evolution of Animal Minds?
Abstract
During the last 50 years, comparative cognition and neurosciences have improved our understanding of animal minds while evolutionary ecology has revealed how selection acts on traits through evolutionary time. We describe how cognition can be subject to natural selection like any other biological trait and how this evolutionary approach can be used to understand the evolution of animal cognition. We recount how comparative and fitness methods have been used to understand the evolution of cognition and outline how these approaches could extend our understanding of cognition. The fitness approach, in particular, offers unprecedented opportunities to study the evolutionary mechanisms responsible for variation in cognition within species and could allow us to investigate both proximate (i.e., neural and developmental) and ultimate (i.e., ecological and evolutionary) underpinnings of animal cognition together. We highlight recent studies that have successfully shown that cognitive traits can be under selection, in particular by linking individual variation in cognition to fitness. To bridge the gap between cognitive variation and fitness consequences and to better understand why and how selection can occur on cognition, we end this review by proposing a more integrative approach to study contemporary selection on cognitive traits combining socio-ecological data, minimally invasive neuroscience methods and measurement of ecologically relevant behaviors linked to fitness. Our overall goal in this review is to build a bridge between cognitive neuroscientists and evolutionary biologists, illustrate how their research could be complementary, and encourage evolutionary ecologists to include explicit attention to cognitive processes in their studies of behavior.
Keywords: brood parasites; cognitive ecology; fitness cost; heredity; individual differences; natural selection; path analysis.
Figures

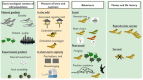
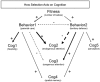
References
-
- Balda R. P., Kamil A. C. (2002). “Spatial and social cognition in corvids: an evolutionary approach,” in The Cognitive Animal: Empirical and Theoretical Perspectives on Animal Cognition eds Bekoff M., Allen C., Burghardt G. M. (Cambridge, MA: MIT Press; ) 129–134.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

 . Neurobiol. Aging 20 447–454. 10.1016/S0197-4580(99)00076-7
-
. Neurobiol. Aging 20 447–454. 10.1016/S0197-4580(99)00076-7
-
