MicroRNA 214 Is a Potential Regulator of Thyroid Hormone Levels in the Mouse Heart Following Myocardial Infarction, by Targeting the Thyroid-Hormone-Inactivating Enzyme Deiodinase Type III
- PMID: 27014189
- PMCID: PMC4783388
- DOI: 10.3389/fendo.2016.00022
MicroRNA 214 Is a Potential Regulator of Thyroid Hormone Levels in the Mouse Heart Following Myocardial Infarction, by Targeting the Thyroid-Hormone-Inactivating Enzyme Deiodinase Type III
Abstract
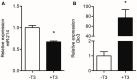
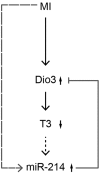
Cardiac thyroid-hormone signaling is a critical determinant of cellular metabolism and function in health and disease. A local hypothyroid condition within the failing heart in rodents has been associated with the re-expression of the fetally expressed thyroid-hormone-inactivating enzyme deiodinase type III (Dio3). While this enzyme emerges as a common denominator in the development of heart failure, the mechanism underlying its regulation remains largely unclear. In the present study, we investigated the involvement of microRNAs (miRNAs) in the regulation of Dio3 mRNA expression in the remodeling left ventricle (LV) of the mouse heart following myocardial infarction (MI). In silico analysis indicated that of the miRNAs that are differentially expressed in the post-MI heart, miR-214 has the highest potential to target Dio3 mRNA. In accordance, a luciferase reporter assay, including the full-length 3'UTR of mouse Dio3 mRNA, showed a 30% suppression of luciferase activity by miR-214. In the post-MI mouse heart, miR-214 and Dio3 protein were shown to be co-expressed in cardiomyocytes, while time-course analysis revealed that Dio3 mRNA expression precedes miR-214 expression in the post-MI LV. This suggests that a Dio3-induced decrease of T3 levels is involved in the induction of miR-214, which was supported by the finding that cardiac miR-214 expression is down regulated by T3 in mice. In vitro analysis of human DIO3 mRNA furthermore showed that miR-214 is able to suppress both mRNA and protein expression. Dio3 mRNA is a target of miR-214 and the Dio3-dependent stimulation of miR-214 expression in post-MI cardiomyocytes supports the involvement of a negative feedback mechanism regulating Dio3 expression.
Keywords: Dio3; SECIS; microRNA; myocardial infarction; thyroid hormone metabolism.
Figures






Similar articles
-
Cardiac expression of deiodinase type 3 (Dio3) following myocardial infarction is associated with the induction of a pluripotency microRNA signature from the Dlk1-Dio3 genomic region.Endocrinology. 2013 Jun;154(6):1973-8. doi: 10.1210/en.2012-2017. Epub 2013 Apr 3. Endocrinology. 2013. PMID: 23554452
-
Left-ventricular remodeling after myocardial infarction is associated with a cardiomyocyte-specific hypothyroid condition.Endocrinology. 2011 Feb;152(2):669-79. doi: 10.1210/en.2010-0431. Epub 2010 Dec 15. Endocrinology. 2011. PMID: 21159857
-
Regulation and function of deiodinases during decidualization in female mice.Endocrinology. 2014 Jul;155(7):2704-17. doi: 10.1210/en.2014-1015. Epub 2014 May 5. Endocrinology. 2014. PMID: 24797630
-
MiR-24-3p/Dio3 axis is essential for BDE47 to induce local thyroid hormone disorder and neurotoxicity.Toxicology. 2023 Jun 1;491:153527. doi: 10.1016/j.tox.2023.153527. Epub 2023 Apr 26. Toxicology. 2023. PMID: 37116683
-
Cardiac Thyroid Hormone Metabolism and Heart Failure.Eur Thyroid J. 2017 Jul;6(3):130-137. doi: 10.1159/000469708. Epub 2017 Apr 21. Eur Thyroid J. 2017. PMID: 28785539 Free PMC article. Review.
Cited by
-
Integrative analysis of differentially expressed genes and miRNAs predicts complex T3-mediated protective circuits in a rat model of cardiac ischemia reperfusion.Sci Rep. 2018 Sep 14;8(1):13870. doi: 10.1038/s41598-018-32237-0. Sci Rep. 2018. PMID: 30218079 Free PMC article.
-
The Impact of Selenium Deficiency on Cardiovascular Function.Int J Mol Sci. 2021 Oct 2;22(19):10713. doi: 10.3390/ijms221910713. Int J Mol Sci. 2021. PMID: 34639053 Free PMC article. Review.
-
Deiodinases and the Three Types of Thyroid Hormone Deiodination Reactions.Endocrinol Metab (Seoul). 2021 Oct;36(5):952-964. doi: 10.3803/EnM.2021.1198. Epub 2021 Oct 21. Endocrinol Metab (Seoul). 2021. PMID: 34674502 Free PMC article. Review.
-
miRNA Expression Profile and Effect of Wenxin Granule in Rats with Ligation-Induced Myocardial Infarction.Int J Genomics. 2017;2017:2175871. doi: 10.1155/2017/2175871. Epub 2017 Aug 15. Int J Genomics. 2017. PMID: 28894747 Free PMC article.
-
Paradigms of Dynamic Control of Thyroid Hormone Signaling.Endocr Rev. 2019 Aug 1;40(4):1000-1047. doi: 10.1210/er.2018-00275. Endocr Rev. 2019. PMID: 31033998 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

