Role of Protein Glycosylation in Candida parapsilosis Cell Wall Integrity and Host Interaction
- PMID: 27014229
- PMCID: PMC4781877
- DOI: 10.3389/fmicb.2016.00306
Role of Protein Glycosylation in Candida parapsilosis Cell Wall Integrity and Host Interaction
Abstract
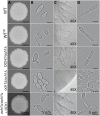
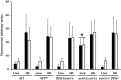
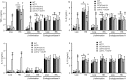
Candida parapsilosis is an important, emerging opportunistic fungal pathogen. Highly mannosylated fungal cell wall proteins are initial contact points with host immune systems. In Candida albicans, Och1 is a Golgi α1,6-mannosyltransferase that plays a key role in the elaboration of the N-linked mannan outer chain. Here, we disrupted C. parapsilosis OCH1 to gain insights into the contribution of N-linked mannosylation to cell fitness and to interactions with immune cells. Loss of Och1 in C. parapsilosis resulted in cellular aggregation, failure of morphogenesis, enhanced susceptibility to cell wall perturbing agents and defects in wall composition. We removed the cell wall O-linked mannans by β-elimination, and assessed the relevance of mannans during interaction with human monocytes. Results indicated that O-linked mannans are important for IL-1β stimulation in a dectin-1 and TLR4-dependent pathway; whereas both, N- and O-linked mannans are equally important ligands for TNFα and IL-6 stimulation, but neither is involved in IL-10 production. Furthermore, mice infected with C. parapsilosis och1Δ null mutant cells had significantly lower fungal burdens compared to wild-type (WT)-challenged counterparts. Therefore, our data are the first to demonstrate that C. parapsilosis N- and O-linked mannans have different roles in host interactions than those reported for C. albicans.
Keywords: Candida parapsilosis; cell wall; host-fungus interplay; mannosylation pathway; mannosyltransferase; virulence.
Figures


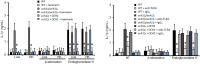


Similar articles
-
Role of Protein Mannosylation in the Candida tropicalis-Host Interaction.Front Microbiol. 2019 Nov 28;10:2743. doi: 10.3389/fmicb.2019.02743. eCollection 2019. Front Microbiol. 2019. PMID: 31849889 Free PMC article.
-
Disruption of Protein Mannosylation Affects Candida guilliermondii Cell Wall, Immune Sensing, and Virulence.Front Microbiol. 2016 Dec 2;7:1951. doi: 10.3389/fmicb.2016.01951. eCollection 2016. Front Microbiol. 2016. PMID: 27994582 Free PMC article.
-
Members of the Candida parapsilosis Complex and Candida albicans are Differentially Recognized by Human Peripheral Blood Mononuclear Cells.Front Microbiol. 2016 Jan 13;6:1527. doi: 10.3389/fmicb.2015.01527. eCollection 2015. Front Microbiol. 2016. PMID: 26793173 Free PMC article.
-
Mannosylation in Candida albicans: role in cell wall function and immune recognition.Mol Microbiol. 2013 Dec;90(6):1147-61. doi: 10.1111/mmi.12426. Epub 2013 Nov 8. Mol Microbiol. 2013. PMID: 24125554 Free PMC article. Review.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
Cited by
-
Presence of O-glycosidically linked oligosaccharides in the cell wall mannan of Candida krusei purified with Benanomicin A.FEBS Open Bio. 2018 Dec 10;9(1):129-136. doi: 10.1002/2211-5463.12558. eCollection 2019 Jan. FEBS Open Bio. 2018. PMID: 30652080 Free PMC article.
-
Candida parapsilosis Cell Wall Proteome Characterization and Effectiveness against Hematogenously Disseminated Candidiasis in a Murine Model.Vaccines (Basel). 2023 Mar 16;11(3):674. doi: 10.3390/vaccines11030674. Vaccines (Basel). 2023. PMID: 36992262 Free PMC article.
-
Silencing of ROT2, the Encoding Gene of the Endoplasmic Reticulum Glucosidase II, Affects the Cell Wall and the Sporothrix schenckii-Host Interaction.J Fungi (Basel). 2022 Nov 18;8(11):1220. doi: 10.3390/jof8111220. J Fungi (Basel). 2022. PMID: 36422041 Free PMC article.
-
Impact of Fungal MAPK Pathway Targets on the Cell Wall.J Fungi (Basel). 2018 Aug 9;4(3):93. doi: 10.3390/jof4030093. J Fungi (Basel). 2018. PMID: 30096860 Free PMC article.
-
Silencing of OCH1 unveils the role of Sporothrix schenckii N-linked glycans during the host-fungus interaction.Infect Drug Resist. 2018 Dec 28;12:67-85. doi: 10.2147/IDR.S185037. eCollection 2019. Infect Drug Resist. 2018. PMID: 30643435 Free PMC article.
References
-
- Brand A., MacCallum D. M., Brown A. J., Gow N. A., Odds F. C. (2004). Ectopic expression of URA3 can influence the virulence phenotypes and proteome of Candida albicans but can be overcome by targeted reintegration of URA3 at the RPS10 locus. Eukaryot Cell 3, 900–909. 10.1128/EC.3.4.900-909.2004 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

