Δ(24)-Sterol Methyltransferase Plays an Important Role in the Growth and Development of Sporothrix schenckii and Sporothrix brasiliensis
- PMID: 27014234
- PMCID: PMC4786573
- DOI: 10.3389/fmicb.2016.00311
Δ(24)-Sterol Methyltransferase Plays an Important Role in the Growth and Development of Sporothrix schenckii and Sporothrix brasiliensis
Abstract
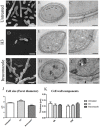
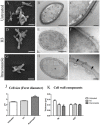
Inhibition of Δ(24)-sterol methyltransferase (24-SMT) in Sporothrix schenckii sensu stricto and Sporothrix brasiliensis was investigated in vitro. The effects on fungal growth and sterol composition of the 24-SMT inhibitor 22-hydrazone-imidazolin-2-yl-chol-5-ene-3β-ol (H3) were compared to those of itraconazole. MIC and MFC analysis showed that H3 was more effective than itraconazole against both species in both their filamentous and yeast forms. H3 showed fungistatic activity in a time-kill assay, with inhibitory activity stronger than that of itraconazole. GC analysis of cell sterol composition showed that sterols present in control cells (ergosterol and precursors) were completely replaced by 14α-methylated sterols after H3 exposure. Itraconazole only partially inhibited ergosterol synthesis but completely arrested synthesis of other sterols found in control cells, promoting accumulation of nine 14α-methyl sterols. Based on these results, we propose a schematic model of sterol biosynthesis pathways in S. schenckii and S. brasiliensis. Effects on cell morphology due to 24-SMT inhibition by H3 as analyzed by SEM and TEM included irregular cell shape, reduced cytoplasmic electron-density, and reduced thickness of the microfibrillar cell wall layer. Moreover, 24-SMT inhibition by H3 promoted mitochondrial disturbance, as demonstrated by alterations in MitoTracker(®) Red CMXRos fluorescence intensity evaluated by flow cytometry. When used in conjunction with itraconazole, H3 enhanced the effectiveness of itraconazole against all tested strains, reducing at least half (or more) the MIC values of itraconazole. In addition, cytotoxicity assays revealed that H3 was more selective toward these fungi than was itraconazole. Thus, 24-SMT inhibition by H3 was an effective antifungal strategy against S. schenckii and S. brasiliensis. Inhibition of the methylation reaction catalyzed by 24-SMT has a strong antiproliferative effect via disruption of ergosterol homeostasis, suggesting that this enzyme is a promising target for novel antifungal therapies against sporotrichosis, either as sole treatments or in combination with itraconazole.
Keywords: Sporothrix brasiliensis; Sporothrix schenckii; antifungal activity; sterol biosynthesis; Δ24-sterol methyltransferase.
Figures




Similar articles
-
Clotrimazole is highly effective in vitro against feline Sporothrix brasiliensis isolates.J Med Microbiol. 2017 Nov;66(11):1573-1580. doi: 10.1099/jmm.0.000608. Epub 2017 Oct 6. J Med Microbiol. 2017. PMID: 28984226
-
Tacrolimus Increases the Effectiveness of Itraconazole and Fluconazole against Sporothrix spp.Front Microbiol. 2017 Sep 15;8:1759. doi: 10.3389/fmicb.2017.01759. eCollection 2017. Front Microbiol. 2017. PMID: 28966608 Free PMC article.
-
Miltefosine is active against Sporothrix brasiliensis isolates with in vitro low susceptibility to amphotericin B or itraconazole.J Med Microbiol. 2015 Apr;64(Pt 4):415-422. doi: 10.1099/jmm.0.000041. Epub 2015 Feb 13. J Med Microbiol. 2015. PMID: 25681323
-
Molecular Components of the Sporothrix schenckii Complex that Induce Immune Response.Curr Microbiol. 2016 Aug;73(2):292-300. doi: 10.1007/s00284-016-1045-5. Epub 2016 Apr 27. Curr Microbiol. 2016. PMID: 27117164 Review.
-
Molecular identification of the Sporothrix schenckii complex.Rev Iberoam Micol. 2014 Jan-Mar;31(1):2-6. doi: 10.1016/j.riam.2013.09.008. Epub 2013 Nov 19. Rev Iberoam Micol. 2014. PMID: 24270070 Review.
Cited by
-
Current Progress on Epidemiology, Diagnosis, and Treatment of Sporotrichosis and Their Future Trends.J Fungi (Basel). 2022 Jul 26;8(8):776. doi: 10.3390/jof8080776. J Fungi (Basel). 2022. PMID: 35893145 Free PMC article. Review.
-
Zinc(II)-Sterol Hydrazone Complex as a Potent Anti-Leishmania Agent: Synthesis, Characterization, and Insight into Its Mechanism of Antiparasitic Action.Pharmaceutics. 2023 Mar 31;15(4):1113. doi: 10.3390/pharmaceutics15041113. Pharmaceutics. 2023. PMID: 37111599 Free PMC article.
-
Adamantylidene-substituted alkylphosphocholine TCAN26 is more active against Sporothrix schenckii than miltefosine.Mem Inst Oswaldo Cruz. 2016 Aug;111(8):523-7. doi: 10.1590/0074-02760160088. Epub 2016 Jul 11. Mem Inst Oswaldo Cruz. 2016. PMID: 27581121 Free PMC article.
-
The Search for Putative Hits in Combating Leishmaniasis: The Contributions of Natural Products Over the Last Decade.Nat Prod Bioprospect. 2021 Oct;11(5):489-544. doi: 10.1007/s13659-021-00311-2. Epub 2021 Jul 14. Nat Prod Bioprospect. 2021. PMID: 34260050 Free PMC article. Review.
-
α-Bisabolol inhibits Aspergillus fumigatus Af239 growth via affecting microsomal ∆24-sterol methyltransferase as a crucial enzyme in ergosterol biosynthesis pathway.World J Microbiol Biotechnol. 2017 Mar;33(3):55. doi: 10.1007/s11274-017-2214-9. Epub 2017 Feb 21. World J Microbiol Biotechnol. 2017. PMID: 28224386
References
-
- Almeida-Paes R., Oliveira M. M. E., Freitas D. F. S., Valle A. C. F., Zancopé-Oliveira R. M., Gutierrez-Galhardo M. C. (2014). Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl. Trop Dis. 8:e3094 10.1371/journal.pntd.0003094 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

