Magnesium Ion Acts as a Signal for Capsule Induction in Cryptococcus neoformans
- PMID: 27014245
- PMCID: PMC4791529
- DOI: 10.3389/fmicb.2016.00325
Magnesium Ion Acts as a Signal for Capsule Induction in Cryptococcus neoformans
Abstract
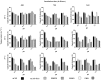
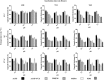
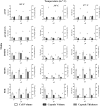
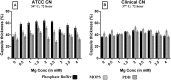
Cryptococcal meningitis caused by Cryptococcus neoformans, is a common opportunistic neural infection in immunocompromised individuals. Cryptococcus meningitis is associated with fungal burden with larger capsule size in cerebrospinal fluid (CSF). To understand the role of CSF constituents in capsule enlargement, we have evaluated the effect of artificial CSF on capsule induction in comparison with various other capsule inducing media. Two different strains of C. neoformans, an environmental and a clinical isolates were used in the present study. While comparing the various capsule inducing media for the two different strains of C. neoformans, it was observed that the capsule growth was significantly increased when grown in artificial CSF at pH 5.5, temperature 34°C for ATCC C. neoformans and 37°C for Clinical C. neoformans and with an incubation period of 72 h. In addition, artificial CSF supports biofilm formation in C. neoformans. While investigating the individual components of artificial CSF, we found that Mg(2+) ions influence the capsule growth in both environmental and clinical strains of C. neoformans. To confirm our results we studied the expression of four major CAP genes namely, CAP10, CAP59, CAP60, and CAP64 in various capsule inducing media and in different concentrations of Mg(2+) and Ca(2+). Our results on gene expression suggest that, Mg(2+) does have an effect on CAP gene expression, which are important for capsule biosynthesis and virulence. Our findings on the role of Mg(2+) ion as a signal for capsule induction will promote a way to elucidate the control mechanisms for capsule biosynthesis in C. neoformans.
Keywords: CAP gene; Cryptococcus neoformans; biofilm; capsule; magnesium ions.
Figures







Similar articles
-
Microreview: capsule-associated genes of Cryptococcus neoformans.Mycopathologia. 2007 Jan;163(1):1-8. doi: 10.1007/s11046-006-0083-0. Mycopathologia. 2007. PMID: 17216326 Review.
-
Expression of capsule-associated genes of Cryptococcus neoformans.Mycopathologia. 2005 Aug;160(1):1-7. doi: 10.1007/s11046-005-0139-6. Mycopathologia. 2005. PMID: 16160761
-
A survey of heterobasidiomycetous yeasts for the presence of the genes homologous to virulence factors of Filobasidiella neoformans, CNLAC1 and CAP59.Microbiology (Reading). 2001 Aug;147(Pt 8):2029-2036. doi: 10.1099/00221287-147-8-2029. Microbiology (Reading). 2001. PMID: 11495981
-
Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans.Infect Immun. 1998 May;66(5):2230-6. doi: 10.1128/IAI.66.5.2230-2236.1998. Infect Immun. 1998. PMID: 9573112 Free PMC article.
-
Immunomodulatory Role of Capsular Polysaccharides Constituents of Cryptococcus neoformans.Front Med (Lausanne). 2019 Jun 19;6:129. doi: 10.3389/fmed.2019.00129. eCollection 2019. Front Med (Lausanne). 2019. PMID: 31275938 Free PMC article. Review.
Cited by
-
Organoselenium Has a Potent Fungicidal Effect on Cryptococcus neoformans and Inhibits the Virulence Factors.Antimicrob Agents Chemother. 2023 Mar 16;67(3):e0075922. doi: 10.1128/aac.00759-22. Epub 2023 Feb 23. Antimicrob Agents Chemother. 2023. PMID: 36815840 Free PMC article.
-
ASK2 Bioactive Compound Inhibits MDR Klebsiella pneumoniae by Antibiofilm Activity, Modulating Macrophage Cytokines and Opsonophagocytosis.Front Cell Infect Microbiol. 2017 Aug 4;7:346. doi: 10.3389/fcimb.2017.00346. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28824881 Free PMC article.
-
Physiological Differences in Cryptococcus neoformans Strains In Vitro versus In Vivo and Their Effects on Antifungal Susceptibility.Antimicrob Agents Chemother. 2017 Feb 23;61(3):e02108-16. doi: 10.1128/AAC.02108-16. Print 2017 Mar. Antimicrob Agents Chemother. 2017. PMID: 28031206 Free PMC article. Review.
-
Investigation of the influence of pH and temperature on melanization and survival under oxidative stress in Cryptococcus neoformans.Arch Microbiol. 2024 Jul 17;206(8):355. doi: 10.1007/s00203-024-04080-7. Arch Microbiol. 2024. PMID: 39017938
-
Exploration of Antifungal and Immunomodulatory Potentials of a Furanone Derivative to Rescue Disseminated Cryptococosis in Mice.Sci Rep. 2017 Nov 13;7(1):15400. doi: 10.1038/s41598-017-15500-8. Sci Rep. 2017. PMID: 29133871 Free PMC article.
References
-
- Alarousy R., Abo H., Yazeed E., Kotb H., Abdalla K., Refai M. (2011). Amplification of capsule-associated genes from Cryptococcus neoformans. N. Y. Sci. J. 4 1–5.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

