Action and Traction: Cytoskeletal Control of Receptor Triggering at the Immunological Synapse
- PMID: 27014258
- PMCID: PMC4779853
- DOI: 10.3389/fimmu.2016.00068
Action and Traction: Cytoskeletal Control of Receptor Triggering at the Immunological Synapse
Abstract
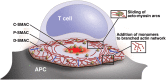
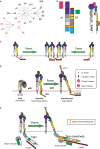
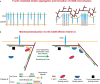
It is well known that F-actin dynamics drive the micron-scale cell shape changes required for migration and immunological synapse (IS) formation. In addition, recent evidence points to a more intimate role for the actin cytoskeleton in promoting T cell activation. Mechanotransduction, the conversion of mechanical input into intracellular biochemical changes, is thought to play a critical role in several aspects of immunoreceptor triggering and downstream signal transduction. Multiple molecules associated with signaling events at the IS have been shown to respond to physical force, including the TCR, costimulatory molecules, adhesion molecules, and several downstream adapters. In at least some cases, it is clear that the relevant forces are exerted by dynamics of the T cell actomyosin cytoskeleton. Interestingly, there is evidence that the cytoskeleton of the antigen-presenting cell also plays an active role in T cell activation, by countering the molecular forces exerted by the T cell at the IS. Since actin polymerization is itself driven by TCR and costimulatory signaling pathways, a complex relationship exists between actin dynamics and receptor activation. This review will focus on recent advances in our understanding of the mechanosensitive aspects of T cell activation, paying specific attention to how F-actin-directed forces applied from both sides of the IS fit into current models of receptor triggering and activation.
Keywords: T cell receptor; actin; adhesion; costimulation; cytoskeleton; immunological synapse; integrin; mechanotransduction.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

